Ann Dermatol.
2016 Aug;28(4):433-437. 10.5021/ad.2016.28.4.433.
Single Low-Dose Radiation Induced Regulation of Keratinocyte Differentiation in Calcium-Induced HaCaT Cells
- Affiliations
-
- 1Department of Dermatology, Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea.
- 2Department of Dermatology, Konkuk University School of Medicine, Seoul, Korea. kjahn@kuh.ac.kr
- 3Korea Institute for Skin and Clinical Sciences and Molecular-Targeted Drug Research Center, Konkuk University, Seoul, Korea.
- KMID: 2344812
- DOI: http://doi.org/10.5021/ad.2016.28.4.433
Abstract
- BACKGROUND
We are continually exposed to low-dose radiation (LDR) in the range 0.1 Gy from natural sources, medical devices, nuclear energy plants, and other industrial sources of ionizing radiation. There are three models for the biological mechanism of LDR: the linear no-threshold model, the hormetic model, and the threshold model.
OBJECTIVE
We used keratinocytes as a model system to investigate the molecular genetic effects of LDR on epidermal cell differentiation.
METHODS
To identify keratinocyte differentiation, we performed western blots using a specific antibody for involucrin, which is a precursor protein of the keratinocyte cornified envelope and a marker for keratinocyte terminal differentiation. We also performed quantitative polymerase chain reaction. We examined whether LDR induces changes in involucrin messenger RNA (mRNA) and protein levels in calcium-induced keratinocyte differentiation.
RESULTS
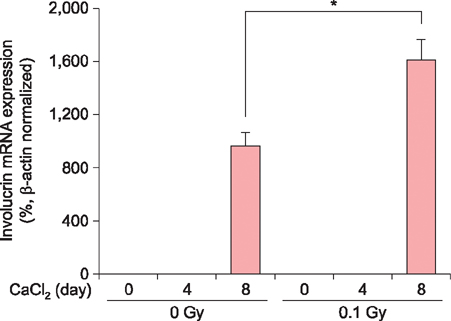
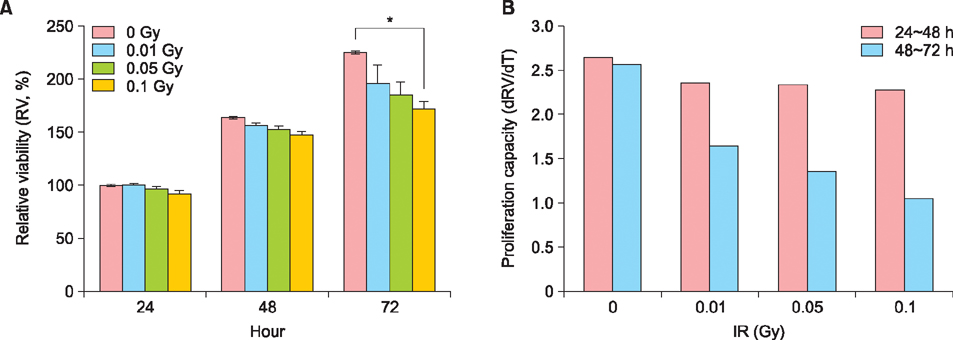
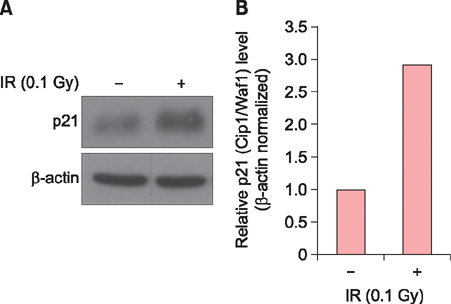
Exposure of HaCaT cells to LDR (0.1 Gy) induced p21 expression. p21 is a key regulator that induces growth arrest and represses stemness, which accelerates keratinocyte differentiation. We correlated involucrin expression with keratinocyte differentiation, and examined the effects of LDR on involucrin levels and keratinocyte development. LDR significantly increased involucrin mRNA and protein levels during calcium-induced keratinocyte differentiation.
CONCLUSION
These studies provide new evidence for the biological role of LDR, and identify the potential to utilize LDR to regulate or induce keratinocyte differentiation.
MeSH Terms
Figure
Reference
-
1. Mobbs SF, Muirhead CR, Harrison JD. Risks from ionising radiation: an HPA viewpoint paper for Safegrounds. J Radiol Prot. 2011; 31:289–307.
Article2. Bae S, Kim K, Cha HJ, Choi Y, Shin SH, An IS, et al. Low-dose γ-irradiation induces dual radio-adaptive responses depending on the post-irradiation time by altering microRNA expression profiles in normal human dermal fibroblasts. Int J Mol Med. 2015; 35:227–237.
Article3. Bae S, Kim K, Cha HJ, Choi Y, Shin SH, An IS, et al. Altered microRNA expression profiles are involved in resistance to low-dose ionizing radiation in the absence of BMI1 in human dermal fibroblasts. Int J Oncol. 2014; 45:1618–1628.
Article4. Liang X, So YH, Cui J, Ma K, Xu X, Zhao Y, et al. The low-dose ionizing radiation stimulates cell proliferation via activation of the MAPK/ERK pathway in rat cultured mesenchymal stem cells. J Radiat Res. 2011; 52:380–386.
Article5. Ahmed KM, Nantajit D, Fan M, Murley JS, Grdina DJ, Li JJ. Coactivation of ATM/ERK/NF-kappaB in the low-dose radiation-induced radioadaptive response in human skin keratinocytes. Free Radic Biol Med. 2009; 46:1543–1550.
Article6. Moskalev AA, Plyusnina EN, Shaposhnikov MV. Radiation hormesis and radioadaptive response in Drosophila melanogaster flies with different genetic backgrounds: the role of cellular stress-resistance mechanisms. Biogerontology. 2011; 12:253–263.
Article7. Seong KM, Kim CS, Seo SW, Jeon HY, Lee BS, Nam SY, et al. Genome-wide analysis of low-dose irradiated male Drosophila melanogaster with extended longevity. Biogerontology. 2011; 12:93–107.
Article8. Ina Y, Sakai K. Activation of immunological network by chronic low-dose-rate irradiation in wild-type mouse strains: analysis of immune cell populations and surface molecules. Int J Radiat Biol. 2005; 81:721–729.
Article9. Eckert RL, Rorke EA. Molecular biology of keratinocyte differentiation. Environ Health Perspect. 1989; 80:109–116.
Article10. Song HJ, Cho CK, Yoo SY, Park KS, Lee YS. Increased induction of Ca2+-mediated differentiation by gamma ray is mediated by endogenous activation of the protein kinase C signaling pathways in mouse epidermal cells. Int J Radiat Oncol Biol Phys. 1998; 41:897–904.
Article11. Barboule N, Lafon C, Chadebech P, Vidal S, Valette A. Involvement of p21 in the PKC-induced regulation of the G2/M cell cycle transition. FEBS Lett. 1999; 444:32–37.
Article12. Cha HJ, Lee OK, Kim SY, Ko JM, Kim SY, Son JH, et al. MicroRNA expression profiling of p-phenylenediamine treatment in human keratinocyte cell line. Mol Cell Toxicol. 2015; 11:19–28.
Article13. Cha HJ, Bae S, Kim K, Kwon SB, An IS, Ahn KJ, et al. Overdosage of methylparaben induces cellular senescence in vitro and in vivo. J Invest Dermatol. 2015; 135:609–612.
Article14. Kwon KJ, Bae S, Kim K, An IS, Ahn KJ, An S, et al. Asiaticoside, a component of Centella asiatica, inhibits melanogenesis in B16F10 mouse melanoma. Mol Med Rep. 2014; 10:503–507.
Article15. Watt FM. Involucrin and other markers of keratinocyte terminal differentiation. J Invest Dermatol. 1983; 81:1 Suppl. 100s–103s.
Article16. Tsukimoto M, Homma T, Ohshima Y, Kojima S. Involvement of purinergic signaling in cellular response to gamma radiation. Radiat Res. 2010; 173:298–309.17. Sudprasert W, Navasumrit P, Ruchirawat M. Effects of low-dose gamma radiation on DNA damage, chromosomal aberration and expression of repair genes in human blood cells. Int J Hyg Environ Health. 2006; 209:503–511.
Article18. Han SK, Song JY, Yun YS, Yi SY. Effect of gamma radiation on cytokine expression and cytokine-receptor mediated STAT activation. Int J Radiat Biol. 2006; 82:686–697.
Article19. Pourfathollah AA, Shaiegan M, Namiri M, Babae GR. Effect of gamma irradiation on lymphocyte proliferation and IL-8 production by lymphocytes isolated from platelet concentrates. Arch Med Res. 2008; 39:590–593.
Article20. Zhang Z, Zhang H, Liu F, Qiu M, Tong J. Effects of gamma radiation on bone-marrow stromal cells. J Toxicol Environ Health A. 2010; 73:514–519.
Article21. Yew TL, Chiu FY, Tsai CC, Chen HL, Lee WP, Chen YJ, et al. Knockdown of p21(Cip1/Waf1) enhances proliferation, the expression of stemness markers, and osteogenic potential in human mesenchymal stem cells. Aging Cell. 2011; 10:349–361.
Article22. Bluwstein A, Kumar N, Léger K, Traenkle J, Oostrum Jv, Rehrauer H, et al. PKC signaling prevents irradiation-induced apoptosis of primary human fibroblasts. Cell Death Dis. 2013; 4:e498.
Article23. Todd C, Reynolds NJ. Up-regulation of p21WAF1 by phorbol ester and calcium in human keratinocytes through a protein kinase C-dependent pathway. Am J Pathol. 1998; 153:39–45.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interleukin-17 and Interleukin-22 Induced Proinflammatory Cytokine Production in Keratinocytes via Inhibitor of Nuclear Factor kappaB Kinase-alpha Expression
- Expressional Changes of c-fos, c-jun, and c-myc Induced by 12-O-tetradecanoylphorbol 13-acetate (TPA) in HaCaT Cells
- In Vitro Effects of 1,25-Dihydroxyvitamin D3 on the Production of Interleukin-1alpha by Ultraviolet B Irradiation in Cultured Human Keratinocyte Cell Line HaCaT Cells
- Differential Expression of TGF-beta Isoforms During Differentiation of HaCaT Human Keratinocyte Cells: Implication for the Separate Role in Epidermal Differentiation
- Baicalein Protects Human Skin Cells against Ultraviolet B-Induced Oxidative Stress