Allergy Asthma Immunol Res.
2014 Mar;6(2):163-168. 10.4168/aair.2014.6.2.163.
Receptor Interacting Protein 2 (RIP2) Is Dispensable for OVA-Induced Airway Inflammation in Mice
- Affiliations
-
- 1Department of Laboratory Animal Medicine, College of Veterinary Medicine, Seoul National University, Seoul, Korea. pjhak@snu.ac.kr
- 2Department of Microbiology and Immunology, School of Medicine, Pusan National University, Yangsan, Korea.
- 3Department of Bio and Brain Engineering, KAIST, Daejeon, Korea.
- 4Department of Biochemistry, College of Medicine, Konyang University, Daejeon, Korea. jonpark@konyang.ac.kr
- KMID: 2260262
- DOI: http://doi.org/10.4168/aair.2014.6.2.163
Abstract
- PURPOSE
Asthma is a pulmonary chronic inflammatory disease characterized by airway obstruction and hyperresponsiveness. Pattern recognition receptors are known to play a key role in the development of allergic diseases as well as host defenses against microbial infection. Receptor interacting protein 2 (RIP2), a serine/threonine kinase, is an adaptor molecule of NOD1 and NOD2, and genetic variation in this receptor is known to be associated with the severity of allergic asthma in children. In this study, we examined the role of RIP2 in the development of allergic airway inflammation in a mouse model.
METHODS
Airway inflammation was induced in mice through intranasal administration of ovalbumin (OVA) after 2 intraperitoneal immunizations with OVA. Lung inflammation and mucus hypersecretion were examined histologically and total cell infiltration in bronchoalveolar (BAL) fluids was determined. Levels of the Th2-related cytokines, IL-5 and IL-13, in lung extracts were measured by ELISA. Serum antigen-specific IgE and IgG1 levels were also assessed.
RESULTS
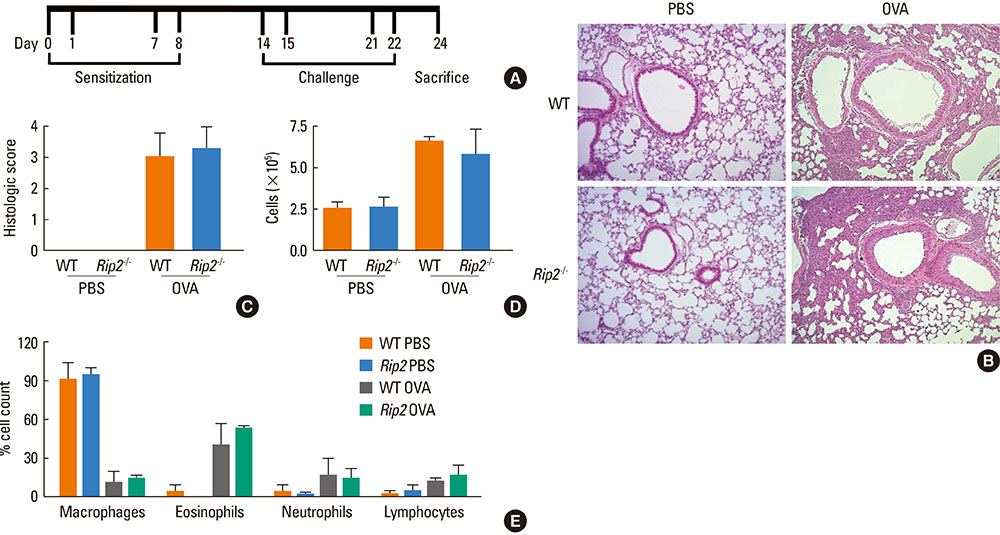
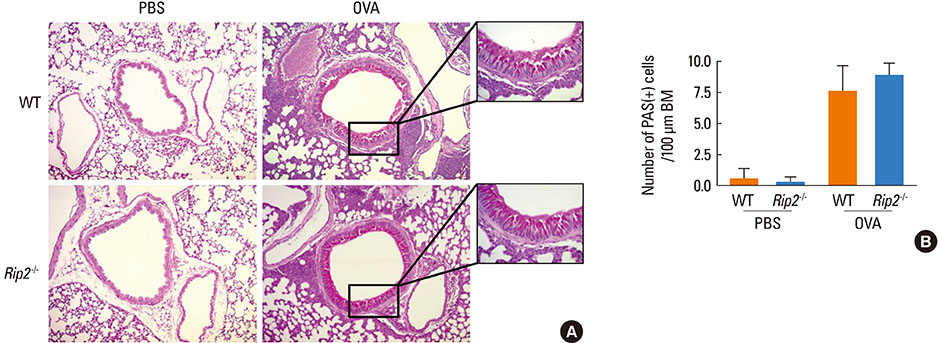
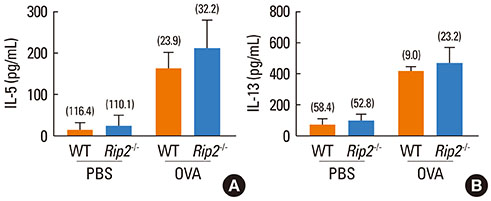
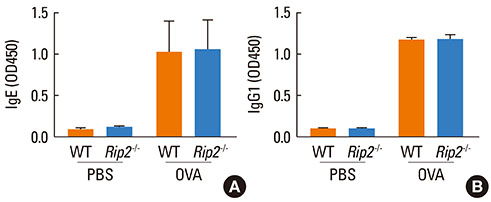
OVA-induced lung inflammation and mucus hypersecretion were not different between WT and RIP2-deficient mice. The IL-5 and IL-13 levels in the bronchoalveolar (BAL) fluids were also not impaired in RIP2-deficient mice compared to WT mice. Moreover, RIP2 deficiency did not affect serum OVA-specific IgG1 and IgE levels.
CONCLUSIONS
Our results suggest that RIP2 is not associated with the development of allergic airway inflammation.
Keyword
MeSH Terms
-
Administration, Intranasal
Airway Obstruction
Animals
Asthma
Child
Cytokines
Enzyme-Linked Immunosorbent Assay
Genetic Variation
Humans
Immunization
Immunoglobulin E
Immunoglobulin G
Inflammation*
Interleukin-13
Interleukin-5
Lung
Methods
Mice*
Mucus
Ovalbumin
Ovum
Phosphotransferases
Pneumonia
Receptors, Pattern Recognition
Cytokines
Immunoglobulin E
Immunoglobulin G
Interleukin-13
Interleukin-5
Ovalbumin
Phosphotransferases
Receptors, Pattern Recognition
Figure
Reference
-
1. Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59:469–478.2. To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet LP. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012; 12:204.3. Busse WW, Lemanske RF Jr. Asthma. N Engl J Med. 2001; 344:350–362.4. Shifren A, Witt C, Christie C, Castro M. Mechanisms of remodeling in asthmatic airways. J Allergy (Cairo). 2012; 2012:316049.5. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012; 18:716–725.6. Nakajima H, Takatsu K. Role of cytokines in allergic airway inflammation. Int Arch Allergy Immunol. 2007; 142:265–273.7. Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998; 282:2258–2261.8. Shakib F, Sihoe J, Smith SJ, Wilding P, Clark MM, Knox A. Circulating levels of IgG1 and IgG4 anti-IgE antibodies and asthma severity. Allergy. 1994; 49:192–195.9. Platts-Mills TA. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med. 2001; 164:S1–S5.10. Kuhl K, Hanania NA. Targeting IgE in asthma. Curr Opin Pulm Med. 2012; 18:1–5.11. Jarjour NN, Kelly EA. Pathogenesis of asthma. Med Clin North Am. 2002; 86:925–936.12. Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Núñez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-kappaB activation. EMBO J. 2008; 27:373–383.13. Inohara N, Koseki T, Lin J, del Peso L, Lucas PC, Chen FF, Ogura Y, Núñez G. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways. J Biol Chem. 2000; 275:27823–27831.14. Kobayashi K, Inohara N, Hernandez LD, Galán JE, Núñez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002; 416:194–199.15. Chen G, Shaw MH, Kim YG, Nuñez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009; 4:365–398.16. Hysi P, Kabesch M, Moffatt MF, Schedel M, Carr D, Zhang Y, Boardman B, von Mutius E, Weiland SK, Leupold W, Fritzsch C, Klopp N, Musk AW, James A, Nunez G, Inohara N, Cookson WO. NOD1 variation, immunoglobulin E and asthma. Hum Mol Genet. 2005; 14:935–941.17. Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrländer C, Nowak D, Holst O, Martinez FD. ALEX-Team. Association between exposure to farming, allergies and genetic variation in CARD4/NOD1. Allergy. 2006; 61:1117–1124.18. Kabesch M, Peters W, Carr D, Leupold W, Weiland SK, von Mutius E. Association between polymorphisms in caspase recruitment domain containing protein 15 and allergy in two German populations. J Allergy Clin Immunol. 2003; 111:813–817.19. Nakashima K, Hirota T, Suzuki Y, Akahoshi M, Shimizu M, Jodo A, Doi S, Fujita K, Ebisawa M, Yoshihara S, Enomoto T, Shirakawa T, Kishi F, Nakamura Y, Tamari M. Association of the RIP2 gene with childhood atopic asthma. Allergol Int. 2006; 55:77–83.20. Moon PD, Choi IH, Kim HM. Naringenin suppresses the production of thymic stromal lymphopoietin through the blockade of RIP2 and caspase-1 signal cascade in mast cells. Eur J Pharmacol. 2011; 671:128–132.21. Mäkelä MJ, Kanehiro A, Dakhama A, Borish L, Joetham A, Tripp R, Anderson L, Gelfand EW. The failure of interleukin-10-deficient mice to develop airway hyperresponsiveness is overcome by respiratory syncytial virus infection in allergen-sensitized/challenged mice. Am J Respir Crit Care Med. 2002; 165:824–831.22. Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012; 18:673–683.23. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010; 140:805–820.24. Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004; 286:L887–L892.25. Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004; 5:975–979.26. Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrländer C, Nowak D, Martinez FD. ALEX Study Team. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004; 113:482–488.27. Redecke V, Häcker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004; 172:2739–2743.28. Torres D, Dieudonné A, Ryffel B, Vilain E, Si-Tahar M, Pichavant M, Lassalle P, Trottein F, Gosset P. Double-stranded RNA exacerbates pulmonary allergic reaction through TLR3: implication of airway epithelium and dendritic cells. J Immunol. 2010; 185:451–459.29. Xirakia C, Koltsida O, Stavropoulos A, Thanassopoulou A, Aidinis V, Sideras P, Andreakos E. Toll-like receptor 7-triggered immune response in the lung mediates acute and long-lasting suppression of experimental asthma. Am J Respir Crit Care Med. 2010; 181:1207–1216.30. Kitagaki K, Businga TR, Kline JN. Oral administration of CpG-ODNs suppresses antigen-induced asthma in mice. Clin Exp Immunol. 2006; 143:249–259.31. Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010; 16:228–231.32. Magalhaes JG, Lee J, Geddes K, Rubino S, Philpott DJ, Girardin SE. Essential role of Rip2 in the modulation of innate and adaptive immunity triggered by Nod1 and Nod2 ligands. Eur J Immunol. 2011; 41:1445–1455.33. Nembrini C, Sichelstiel A, Kisielow J, Kurrer M, Kopf M, Marsland BJ. Bacterial-induced protection against allergic inflammation through a multicomponent immunoregulatory mechanism. Thorax. 2011; 66:755–763.34. Hall HT, Wilhelm MT, Saibil SD, Mak TW, Flavell RA, Ohashi PS. RIP2 contributes to Nod signaling but is not essential for T cell proliferation, T helper differentiation or TLR responses. Eur J Immunol. 2008; 38:64–72.35. Conrad ML, Ferstl R, Teich R, Brand S, Blümer N, Yildirim AO, Patrascan CC, Hanuszkiewicz A, Akira S, Wagner H, Holst O, von Mutius E, Pfefferle PI, Kirschning CJ, Garn H, Renz H. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med. 2009; 206:2869–2877.36. Kim YG, Park JH, Reimer T, Baker DP, Kawai T, Kumar H, Akira S, Wobus C, Núñez G. Viral infection augments Nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe. 2011; 9:496–507.37. Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Núñez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008; 28:246–257.38. Park JH, Kim YG, Núñez G. RICK promotes inflammation and lethality after gram-negative bacterial infection in mice stimulated with lipopolysaccharide. Infect Immun. 2009; 77:1569–1578.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of CpG-oligodeoxynucleotides in Chronic Inflammation and Remodeling of Airway in a Murine Model of Bronchial Asthma
- Human Cytotoxic T Lymphocyte-associated Antigen 4-immunoglobulin Fusion Protein Inhibits Airway Inflammation and Hyperresponsiveness in a Murine Model of Asthma
- N-acetylcysteine decreases airway inflammation and responsiveness in asthma by modulating claudin 18 expression
- Administration of Pigment Epithelium-Derived Factor Inhibits Airway Inflammation and Remodeling in Chronic OVA-Induced Mice via VEGF Suppression
- Effect of intranasal rosiglitazone on airway inflammation and remodeling in a murine model of chronic asthma