Allergy Asthma Immunol Res.
2016 Mar;8(2):161-169. 10.4168/aair.2016.8.2.161.
Administration of Pigment Epithelium-Derived Factor Inhibits Airway Inflammation and Remodeling in Chronic OVA-Induced Mice via VEGF Suppression
- Affiliations
-
- 1Department of Respiratory Medicine, The First Affiliated Hospital, Nanjing Medical University, Nanjing, China.
- 2Department of Respiratory Medicine, The Second Affiliated Hospital, Nanjing Medical University, Nanjing, China. jingshuyue@163.com
- KMID: 2165933
- DOI: http://doi.org/10.4168/aair.2016.8.2.161
Abstract
- PURPOSE
Pigment epithelium-derived factor (PEDF) is a recently discovered antiangiogenesis protein. PEDF possesses powerful anti-inflammatory, antioxidative, antiangiogenic, and antifibrosis properties. It has been reported that PEDF can regulate vascular endothelial growth factor (VEGF) expression. This study aimed to evaluate whether recombinant PEDF protein could attenuate allergic airway inflammation and airway remodeling via the negative regulation of VEGF using a murine model of chronic ovalbumin (OVA)-induced asthma and BEAS-2B human bronchial epithelial cells.
METHODS
In an in vivo experiment, mice sensitized with OVA were chronically airway challenged with aerosolized 1% OVA solution for 8 weeks. Treated mice were given injections of recombinant PEDF protein (50 or 100 microg/kg body weight) via the tail vein. In an in vitro experiment, we investigated the effects of recombinant PEDF protein on VEGF release levels in BEAS-2B cells stimulated with IL-1beta.
RESULTS
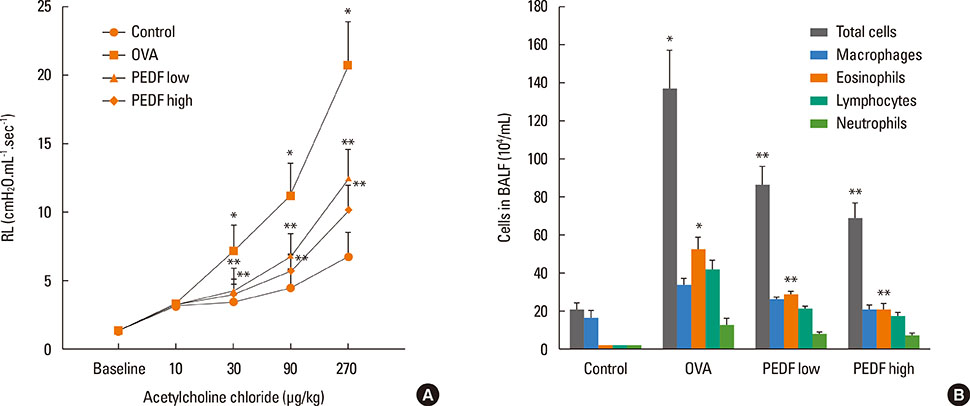
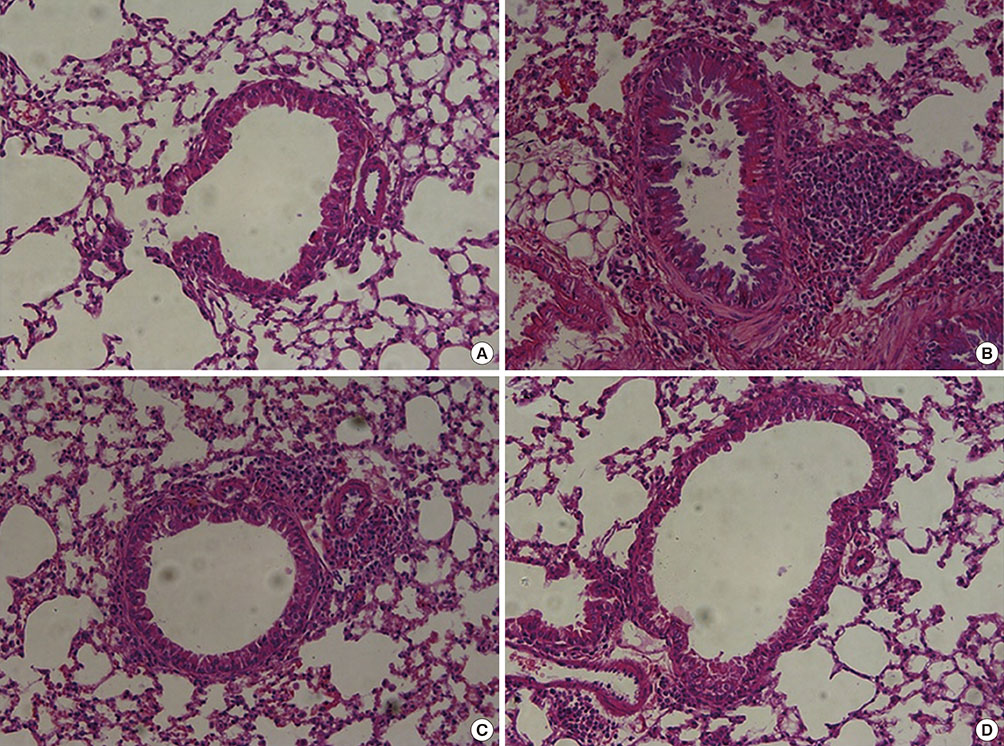
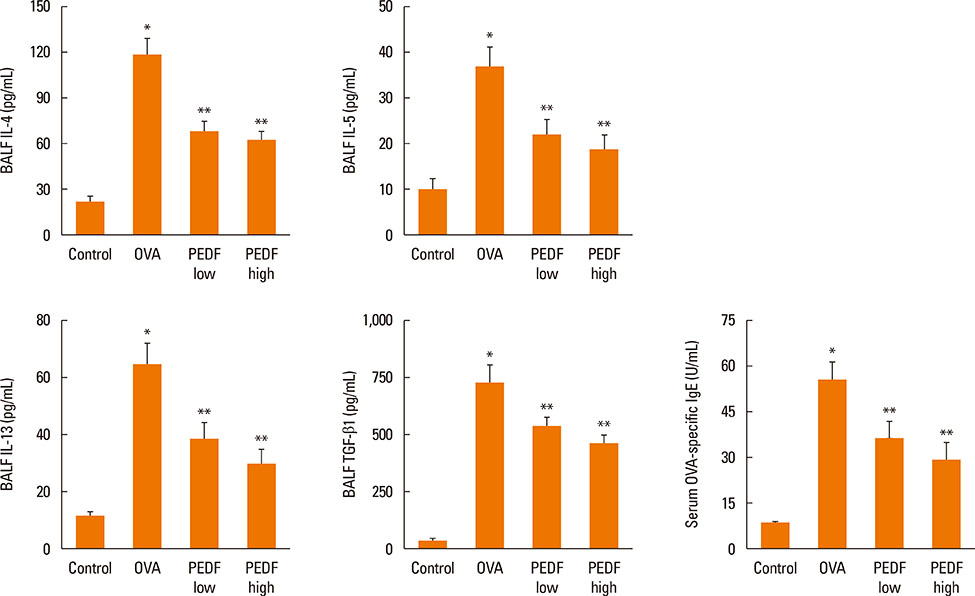
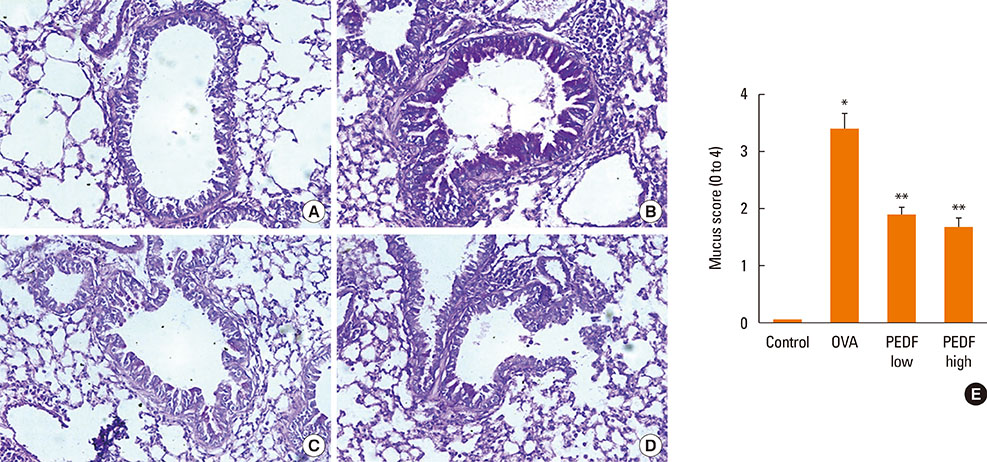
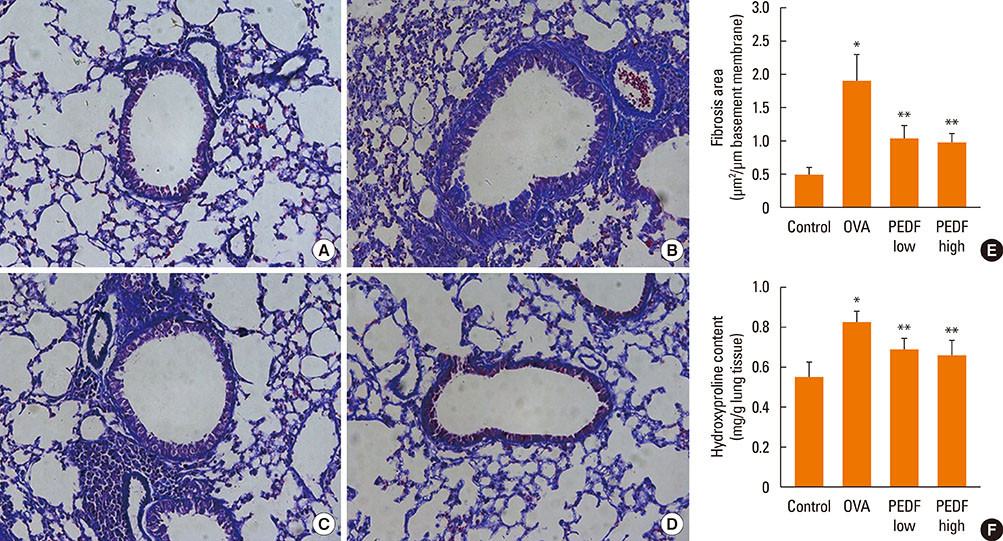
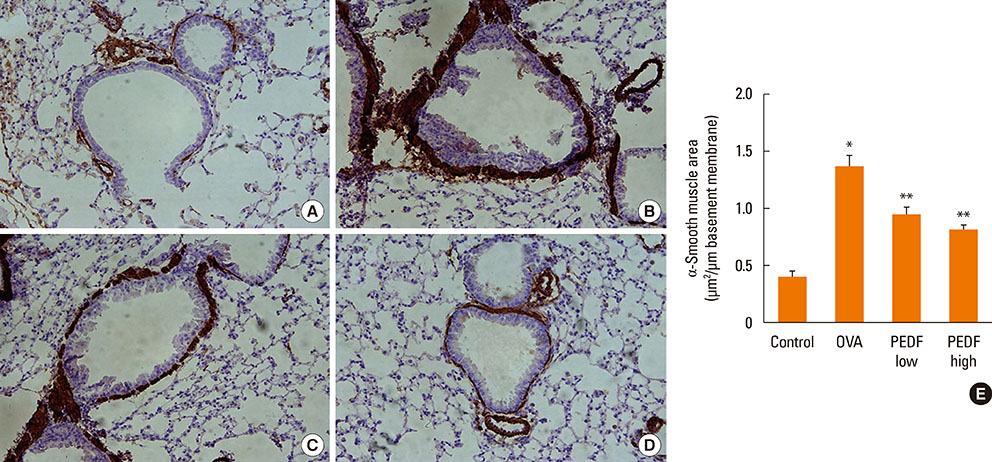
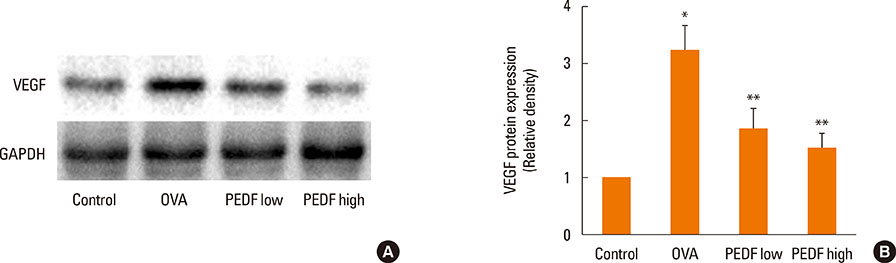
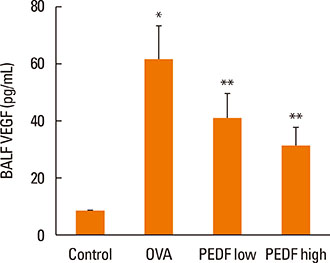
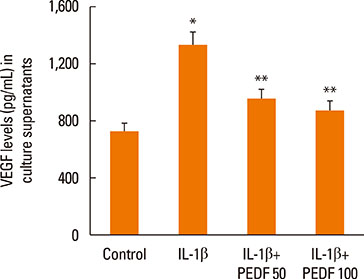
Recombinant PEDF protein significantly inhibited eosinophilic airway inflammation, airway hyperresponsiveness, and airway remodeling, including goblet cell hyperplasia, subepithelial collagen deposition, and airway smooth muscle hypertrophy. In addition, recombinant PEDF protein suppressed the enhanced expression of VEGF protein in lung tissue and bronchoalveolar lavage fluid (BALF) in OVA-challenged chronically allergic mice. In the in vitro experiment, VEGF expression was increased after IL-1beta stimulation. Pretreatment with 50 and 100 ng/mL of recombinant PEDF protein significantly attenuated the increase in VEGF release levels in a concentration-dependent manner in BEAS-2B cells stimulated by IL-1beta.
CONCLUSIONS
These results suggest that recombinant PEDF protein may abolish the development of characteristic features of chronic allergic asthma via VEGF suppression, providing a potential treatment option for chronic airway inflammation diseases such as asthma.
Keyword
MeSH Terms
Figure
Reference
-
1. Awad AS, Gao T, Gvritishvili A, You H, Liu Y, Cooper TK, et al. Protective role of small pigment epithelium-derived factor (PEDF) peptide in diabetic renal injury. Am J Physiol Renal Physiol. 2013; 305:F891–F900.2. Tsai TH, Shih SC, Ho TC, Ma HI, Liu MY, Chen SL, et al. Pigment epithelium-derived factor 34-mer peptide prevents liver fibrosis and hepatic stellate cell activation through down-regulation of the PDGF receptor. PLoS One. 2014; 9:e95443.3. Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006; 20:323–325.4. Liu X, Chen HH, Zhang LW. Potential therapeutic effects of pigment epithelium-derived factor for treatment of diabetic retinopathy. Int J Ophthalmol. 2013; 6:221–227.5. Ide Y, Matsui T, Ishibashi Y, Takeuchi M, Yamagishi S. Pigment epithelium-derived factor inhibits advanced glycation end product-elicited mesangial cell damage by blocking NF-kappaB activation. Microvasc Res. 2010; 80:227–232.6. Alcantara MB, Dass CR. Pigment epithelium-derived factor as a natural matrix metalloproteinase inhibitor: a comparison with classical matrix metalloproteinase inhibitors used for cancer treatment. J Pharm Pharmacol. 2014; 66:895–902.7. Kang HJ, Park HH, Chae SW, Hwang SJ, Lee SH, Lee SH, et al. Increased expression of pigment epithelium-derived factor in allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2008; 134:1094–1098.8. Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011; 128:451–462.9. Paik SH, Kim WK, Park JS, Park CS, Jin GY. A quantitative study of airway changes on micro-CT in a mouse asthma model: comparison with histopathological findings. Allergy Asthma Immunol Res. 2014; 6:75–82.10. Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007; 19:676–680.11. Koyama S, Sato E, Tsukadaira A, Haniuda M, Numanami H, Kurai M, et al. Vascular endothelial growth factor mRNA and protein expression in airway epithelial cell lines in vitro. Eur Respir J. 2002; 20:1449–1456.12. Chuderland D, Ben-Ami I, Bar-Joseph H, Shalgi R. Role of pigment epithelium-derived factor in the reproductive system. Reproduction. 2014; 148:R53–R61.13. Seki R, Yamagishi S, Matsui T, Yoshida T, Torimura T, Ueno T, et al. Pigment epithelium-derived factor (PEDF) inhibits survival and proliferation of VEGF-exposed multiple myeloma cells through its anti-oxidative properties. Biochem Biophys Res Commun. 2013; 431:693–697.14. Ueda S, Yamagishi S, Matsui T, Jinnouchi Y, Imaizumi T. Administration of pigment epithelium-derived factor inhibits left ventricular remodeling and improves cardiac function in rats with acute myocardial infarction. Am J Pathol. 2011; 178:591–598.15. Zha WJ, Qian Y, Shen Y, Du Q, Chen FF, Wu ZZ, et al. Galangin abrogates ovalbumin-induced airway inflammation via negative regulation of NF-κB. Evid Based Complement Alternat Med. 2013; 2013:767689.16. Du Q, Feng GZ, Shen L, Cui J, Cai JK. Paeonol attenuates airway inflammation and hyperresponsiveness in a murine model of ovalbumin-induced asthma. Can J Physiol Pharmacol. 2010; 88:1010–1016.17. Du Q, Zhou LF, Chen Z, Gu XY, Huang M, Yin KS. Imiquimod, a toll-like receptor 7 ligand, inhibits airway remodelling in a murine model of chronic asthma. Clin Exp Pharmacol Physiol. 2009; 36:43–48.18. Yang J, Chen S, Huang X, Han J, Wang Q, Shi D, et al. Growth suppression of cervical carcinoma by pigment epithelium-derived factor via anti-angiogenesis. Cancer Biol Ther. 2010; 9:967–974.19. Rogers ME, Navarro ID, Perkumas KM, Niere SM, Allingham RR, Crosson CE, et al. Pigment epithelium-derived factor decreases outflow facility. Invest Ophthalmol Vis Sci. 2013; 54:6655–6661.20. Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, et al. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994; 24:73–80.21. Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001; 167:4668–4675.22. Mishra A, Weaver TE, Beck DC, Rothenberg ME. Interleukin-5-mediated allergic airway inflammation inhibits the human surfactant protein C promoter in transgenic mice. J Biol Chem. 2001; 276:8453–8459.23. Liou CJ, Cheng PY, Huang WC, Chan CC, Chen MC, Kuo ML, et al. Oral lovastatin attenuates airway inflammation and mucus secretion in ovalbumin-induced murine model of asthma. Allergy Asthma Immunol Res. 2014; 6:548–557.24. Kim TH, Park YM, Ryu SW, Kim DJ, Park JH, Park JH. Receptor interacting protein 2 (RIP2) is dispensable for OVA-induced airway inflammation in mice. Allergy Asthma Immunol Res. 2014; 6:163–168.25. Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol. 1998; 102:783–788.26. Mao T, Gao L, Li H, Li J. Pigment epithelium-derived factor inhibits high glucose induced oxidative stress and fibrosis of cultured human glomerular mesangial cells. Saudi Med J. 2011; 32:769–777.27. Schmitz JC, Protiva P, Gattu AK, Utsumi T, Iwakiri Y, Neto AG, et al. Pigment epithelium-derived factor regulates early pancreatic fibrotic responses and suppresses the profibrotic cytokine thrombospondin-1. Am J Pathol. 2011; 179:2990–2999.28. Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol Cell Biol. 2007; 85:348–356.29. Martin JG, Duguet A, Eidelman DH. The contribution of airway smooth muscle to airway narrowing and airway hyperresponsiveness in disease. Eur Respir J. 2000; 16:349–354.30. Meyer N, Akdis CA. Vascular endothelial growth factor as a key inducer of angiogenesis in the asthmatic airways. Curr Allergy Asthma Rep. 2013; 13:1–9.31. Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004; 10:1095–1103.32. Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express proremodeling factors. J Allergy Clin Immunol. 2012; 129:990.e6–997.e6.33. Asai K, Kanazawa H, Kamoi H, Shiraishi S, Hirata K, Yoshikawa J. Increased levels of vascular endothelial growth factor in induced sputum in asthmatic patients. Clin Exp Allergy. 2003; 33:595–599.34. Chetta A, Zanini A, Foresi A, D'Ippolito R, Tipa A, Castagnaro A, et al. Vascular endothelial growth factor up-regulation and bronchial wall remodelling in asthma. Clin Exp Allergy. 2005; 35:1437–1442.35. Zou H, Fang QH, Ma YM, Wang XY. Analysis of growth factors in serum and induced sputum from patients with asthma. Exp Ther Med. 2014; 8:573–578.36. Yuksel H, Yilmaz O, Karaman M, Bagriyanik HA, Firinci F, Kiray M, et al. Role of vascular endothelial growth factor antagonism on airway remodeling in asthma. Ann Allergy Asthma Immunol. 2013; 110:150–155.37. Zhang SX, Wang JJ, Gao G, Parke K, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006; 37:1–12.38. Johnston EK, Francis MK, Knepper JE. Recombinant pigment epithelium-derived factor PEDF binds vascular endothelial growth factor receptors 1 and 2. In Vitro Cell Dev Biol Anim. 2015; 51:730–738.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of CpG-oligodeoxynucleotides in Chronic Inflammation and Remodeling of Airway in a Murine Model of Bronchial Asthma
- Effect of intranasal rosiglitazone on airway inflammation and remodeling in a murine model of chronic asthma
- Different anti-remodeling effect of nilotinib and fluticasone in a chronic asthma model
- Inhibitory Effects of Resveratrol on Airway Remodeling by Transforming Growth Factor-β/Smad Signaling Pathway in Chronic Asthma Model
- Effects of Korean Red Ginseng Extracts on Airway Hyperresponsiveness and Inflammation in a Murine Asthma Model