Korean Circ J.
2008 Feb;38(2):110-118. 10.4070/kcj.2008.38.2.110.
Effect of Adult Bone Marrow Stem Cells on Myocardial Regeneration in Doxorubicin-Induced Mouse Cardiomyopathy
- Affiliations
-
- 1Cardiovascular Medicine, Department of Internal Medicine, The Catholic University of Korea, College of Medicine, Seoul, Korea. whitesh@catholic.ac.kr
- KMID: 2225848
- DOI: http://doi.org/10.4070/kcj.2008.38.2.110
Abstract
- BACKGROUND AND OBJECTIVES
Bone marrow cells have been shown to differentiate into various cell lineages, including cardiomyocytes, in recent studies. This study evaluates the hypothesis that intravenous injection of bone marrow mononuclear cells (BMNCs) into rats with doxorubicin-induced cardiomyopathy can induce myocardial regeneration and improve myocardial contractility.
MATERIALS AND METHODS
Adult male Sprague-Dawley rats were induced to develop cardiomyopathy by treatment with doxorubicin (2.5 mg/kg, 6 times, 2-week period). Stem cell enriched BMNCs were injected into the tail vein of the rats after cessation of the doxorubicin injections. One week after the injection of PKH-67-labeled BMNCs, the localization of transplanted cells was evaluated. Immunohistochemical studies and Western blots were performed two weeks after BMNCs injection.
RESULTS
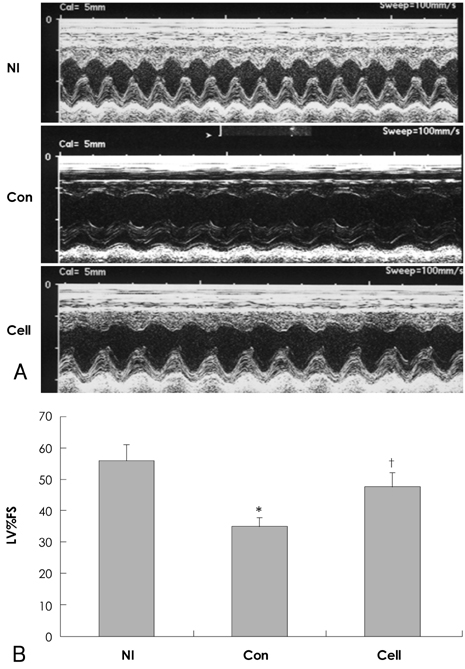
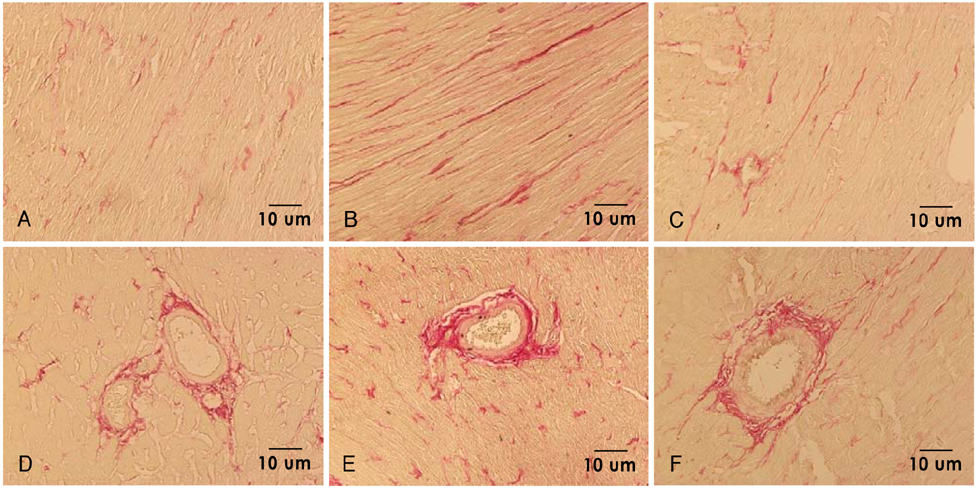
Cell-treated animals showed significant improvement in left ventricular fractional shortening as compared to untreated (control) rats (cell treated group vs. control group 47.2+/-4.9% vs. 34.4+/-3.6%, p<0.01). Histological analyses showed that in the cell-treated animals there was an increase in ventricular interstitial collagen deposition and the cell-treated animals had an improved number of capillary endothelial cells as compared with the control rats. PKH-67-labeled BMNCs and cell proliferation by BrdU was noted in the cell-treated hearts. Cardiac CXCR4 protein expression increased at day 7 and 14 in the cell-treated rats, but only at day 14 in the control animals.
CONCLUSION
These results suggest that intravenous injection of BMNCs effectively induce engraftment of BMNCs into the myocardium and attenuation of fibrosis. Intravenous injection of BMNCs also improved myocardial contractility in doxorubicininduced cardiomyopathy.
Keyword
MeSH Terms
-
Adult
Adult Stem Cells
Animals
Blotting, Western
Bone Marrow
Bone Marrow Cells
Bromodeoxyuridine
Cardiomyopathies
Cell Lineage
Cell Proliferation
Collagen
Doxorubicin
Endothelial Cells
Fibrosis
Heart
Humans
Injections, Intravenous
Male
Mice
Myocardium
Myocytes, Cardiac
Rats
Rats, Sprague-Dawley
Regeneration
Stem Cells
Transplants
Veins
Bromodeoxyuridine
Collagen
Doxorubicin
Figure
Reference
-
1. Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977. 197:165–167.2. Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998. 339:900–905.3. Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001. 344:1750–1757.4. Quaini F, Urbanek K, Beltrami AP, et al. Chimerism of the transplanted heart. N Engl J Med. 2002. 346:5–15.5. Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001. 107:1395–1402.6. Perin EC, Dohmann HF, Borojevic R, et al. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy. Circulation. 2004. 110(11 Suppl 1):II213–II218.7. Baek SH. The current concept of cell therapy for heart failure. Korean Circ J. 2005. 35:415–423.8. Korbling M, Estrov Z. Adult stem cell for tissue repair: a new therapeutic concept? N Engl J Med. 2003. 349:570–582.9. Park CS, Youn HJ, Cho EJ, et al. Cardioprotective effect of IGF-1 in mouse with adriamycin induced cardiomyopathy. Korean Circ J. 2002. 32:1116–1123.10. Tomita M, Adachi Y, Yamada H, et al. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells. 2002. 20:279–283.11. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979. 11:447–455.12. Soonpaa MH, Koh GY, Klug MG, Field LJ. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994. 264:98–101.13. Taylor DA, Atkins BZ, Hungspreugs P, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998. 4:929–933.14. Lim SY, Jeong MH, Ahn YK, et al. The effects of mesenchymal stem cells transduced with akt in a porcine myocardial infarction model. Korean Circ J. 2005. 35:734–741.15. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.16. Orlic D, Kajstura J, Chimenti S, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001. 98:10344–10349.17. Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000. 97:3422–3427.18. Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001. 103:2776–2779.19. Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002. 90:284–288.20. Tomita S, Mickle DA, Weisel RD, et al. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg. 2002. 123:1132–1140.21. Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003. 107:2134–2139.22. Peled A, Kollet O, Ponomaryov T, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000. 95:3289–3296.23. Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002. 3:687–694.24. Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996. 382:635–638.25. Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003. 111:187–196.26. Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998. 393:591–594.27. Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003. 107:1322–1328.28. Doitsidou M, Reichman-Fried M, Stebler J, et al. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002. 111:647–659.29. Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998. 393:595–599.30. Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001. 97:3354–3360.