Korean Circ J.
2009 Mar;39(3):87-92. 10.4070/kcj.2009.39.3.87.
Is Stem Cell-Based Therapy Going on or Out for Cardiac Disease?
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Kwandong University College of Medicine, Goyang, Korea. cardio101@daum.net
- 2Division of Cardiology, Department of Medicine, Emory University School of Medicine, Atlanta, USA.
- KMID: 1825963
- DOI: http://doi.org/10.4070/kcj.2009.39.3.87
Abstract
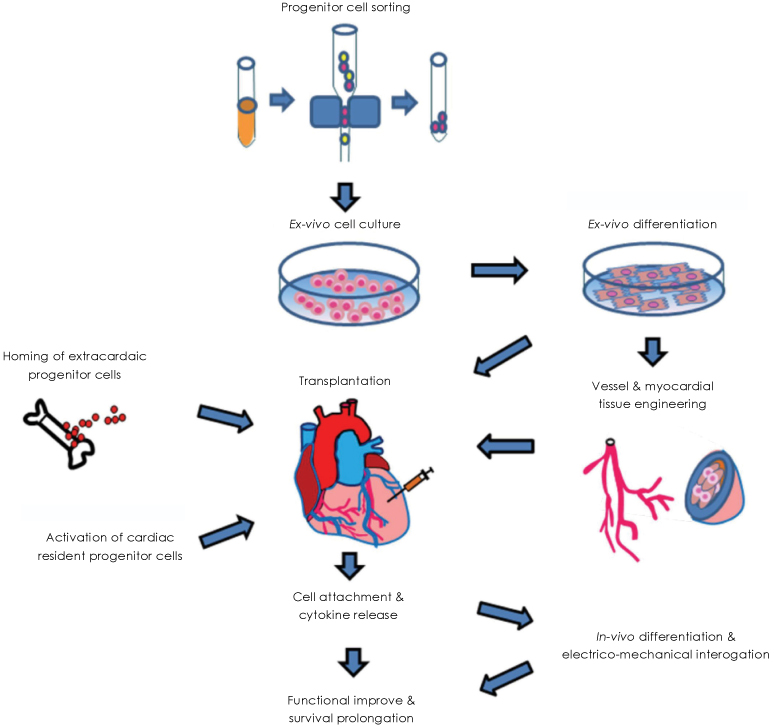
- Acute myocardial infarction and subsequent heart failure are leading causes of death worldwide. Stem cell-based therapies have improved cardiac function in recent clinical trials, but cardiomyocyte regeneration has not been demonstrated in human hearts. Angiogenesis and restoration of cardiac perfusion have been successfully performed using bone marrow derived stem cells and other adult stem cells. Resident cardiac stem cells are known to differentiate into multiple heart cell types, including cardiomyocytes. Furthermore, induced pluripotent stem cells are a focus of research due to the great potential for customized stem cell therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008. 453:322–329.2. Jolicoeur EM, Granger CB, Fakunding JL, et al. Bringing cardiovascular cell-based therapy to clinical application: perspectives based on a National Heart, Lung, and Blood Institute Cell Therapy Working Group meeting. Am Heart J. 2007. 153:732–742.3. Beltrami AP, Urbanek K, Anversa P, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001. 344:1750–1757.4. Quaini F, Urbanek K, Anversa P, et al. Chimerism of the transplanted heart. N Engl J Med. 2002. 346:5–15.5. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999. 9:10711–10716.6. Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001. 107:1395–1402.7. Wu SM, Chien KR, Mummery C. Origins and fates of cardiovascular progenitor cells. Cell. 2008. 132:537–543.8. Yuasa S, Itabashi Y, Koshimizu U, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005. 23:607–611.9. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998. 282:1145–1147.10. Orlic D, Kajstura J, Anversa P, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.11. Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004. 364:141–148.12. Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006. 355:1210–1221.13. Assmus B, Honold J, Schächinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006. 355:1222–1232.14. Adler ED, Maddox TM. Cell therapy for cardiac disease: where do we go from here? Nat Clin Pract Cardiovasc Med. 2007. 4:2–3.15. Lunde K, Solheim S, Aakhus S, et al. Exercise capacity and quality of life after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction. Am Heart J. 2007. 154:710.e1–710.e8.16. Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004. 428:664–668.17. Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004. 428:668–673.18. Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004. 10:494–501.19. Schächinger V, Tonn T, Dimmeler S, Zeiher AM. Bone-marrow-derived progenitor cell therapy in need of proof of concept: design of the REPAIR-AMI trial. Nat Clin Pract Cardiovasc Med. 2006. 3:Suppl 1. S23–S28.20. Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007. 167:989–997.21. Messina E, De Angelis L, Giacomello A, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004. 95:911–921.22. Bearzi C, Rota M, Anversa P, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007. 104:14068–14073.23. Hudon-David F, Bouzeghrane F, Couture P, Thibault G. Thy-1 expression by cardiac fibroblasts: lack of association with myofibroblast contractile markers. J Mol Cell Cardiol. 2007. 42:991–1000.24. Smart N, Risebro CA, Melville AA, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007. 445:177–182.25. Lepilina A, Coon AN, Poss KD, et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006. 127:607–619.26. Lepilina A, Coon AN, Kikuchi K, et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006. 127:607–619.27. Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008. 451:937–942.28. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006. 126:663–676.29. Yu J, Vodyanik MA, Thomson JA, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007. 318:1917–1920.30. Hanna J, Wernig M, Jaenisch R, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007. 318:1920–1923.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Concepts in Stem Cell Therapy for Cardiovascular Diseases: What We Know and Don't Know
- The Role of Large Animal Studies in Cardiac Regenerative Therapy Concise Review of Translational Stem Cell Research
- Current Status of G-CSF Based Stem Cell Therapy for Patients with Myocardial Infarction
- Cell Biological Characteristics of Adult Stem Cells
- Clinical application of stem cell in cardiovascular diseases