J Korean Med Assoc.
2010 Sep;53(9):780-790. 10.5124/jkma.2010.53.9.780.
Diagnosis and treatment of allergic rhinitis
- Affiliations
-
- 1Department of Otorhinolaryngology, Yonsei University College of Medicine, Seoul, Korea. ydrhinol@yuhs.ac
- KMID: 2188374
- DOI: http://doi.org/10.5124/jkma.2010.53.9.780
Abstract
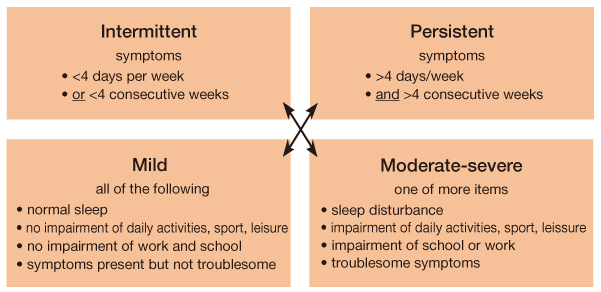
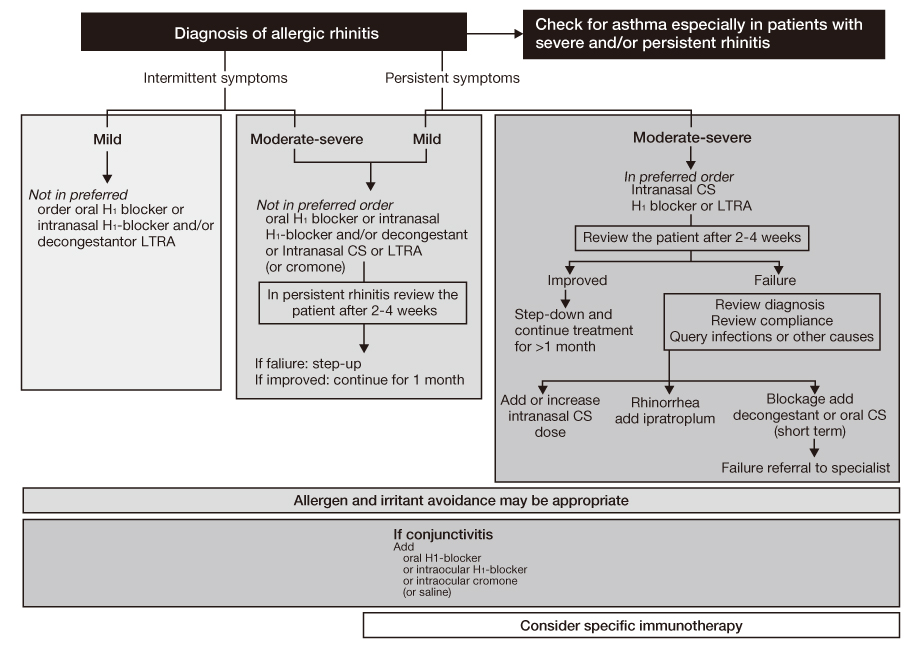
- Allergic rhinitis (AR) is a global health problem affecting at least 10 to 25% of the population, and is a chronic respiratory illness that affects quality of life, productivity, and other co-morbid conditions such as asthma and sinusitis. Classification of AR has been changed to intermittent/persistent (duration) and mild/moderate-severe (severity) based on the "Allergic Rhinitis and Its Impact on Asthma" (ARIA) workshop report published in 2001. A patient's history and skin prick test results are of utmost importance for its diagnosis regardless of classification system. Treatment should be based on the patient's age, severity, and duration of symptoms. The treatment algorithm has recently been revised by 2008 ARIA guidelines. Treatment options for AR consist of allergen avoidance, pharmacotherapy, immunotherapy, and surgery. Patients should be advised to avoid known allergens and educate themselves about their condition. Although allergen avoidance and immunotherapy are theoretically ideal, intranasal corticosteroids are the most effective treatment in persistent and moderate-severe AR. Sublingual immunotherapy has been introduced and has shown good results in its efficacy and safety. Physicians are advised to be alert to the state-of-the-art knowledge on AR and be willing to take advantage of recent progress on AR.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Molecular Identification of Schizophyllum commune as a Cause of Allergic Fungal Sinusitis
Eun Jeong Won, Jong Hee Shin, Sang Chul Lim, Myung Geun Shin, Soon Pal Suh, Dong Wook Ryang
Ann Lab Med. 2012;32(5):375-379. doi: 10.3343/alm.2012.32.5.375.
Reference
-
1. Cockburn IM, Bailit HL, Berndt ER, Finkelstein SN. Loss of work productivity due to illness and medical treatment. J Occup Environ Med. 1999. 41:948–953.
Article2. Jee HM, Kim KW, Kim CS, Sohn MH, Shin DC, Kim KE. Prevalence of asthma, rhinitis, and eczema in Korean children using the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaires. Pediatr Allergy Respir Dis. 2009. 19:165–172.3. Bjorksten B, Clayton T, Stewart A, Strachan D. Worldwild time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008. 19:110–124.
Article4. Bousquet J, Van Cauwenberge P, Khaltaev N. Aria Workshop Group. World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001. 108:5 Suppl. S147–S334.
Article5. Salib RJ, Lau LC, Howarth PH. The novel use of the human nasal epithelial cell line RPMI 2650 as an in vitro model to study the influence of allergens and cytokines on transforming growth factor-beta gene expression and protein release. Clin Exp Allergy. 2005. 35:811–819.
Article6. Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol. 2010. 125:S284–S296.
Article7. Min YG. The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2010. 2:65–76.
Article8. Jung YG, Cho HJ, Park GY, Min JY, Kim HY, Dhong HJ, Chung SK, Kim SW. Comparison of the skin-prick test and Phadia ImmunoCAP as tools to diagnose house-dust mite allergy. Am J Rhinol Allergy. 2010. 24:226–229.
Article9. Bang JH, Kim YJ, Shin HS, Lee BJ. Clinical analysis of allergic rhinitis in Seoul. J Rhinol. 1996. 3:130–134.10. Heo Y, Kim HA. Correlation between skin prick test and enzyme-linked immunosorbent assay using serum for identification of subjects positive to major indoor respiratory allergen. J Env Health Sci. 2008. 34:369–373.
Article11. Larenas-Linnemann D, Matta JJ, Shah-Hosseini K, Michels A, Mösges R. Skin prick test evaluation of Dermatophagoides pteronyssinus diagnostic extracts from Europe, Mexico, and the United States. Ann Allergy Asthma Immunol. 2010. 104:420–425.
Article12. Nelson HS. Diagnostic procedures in allergy. I. allergy skin testing. Ann Allergy. 1983. 51:411–418.13. Jang TY, Choi JC, Jung DH. Detection of Specific IgE Antibody from Nasal Secretion in Allergic Rhinitis. Korean J Otolaryngol-Head Neck Surg. 2000. 43:1318–1322.14. Han MY, Jee JM, Kim HY, Lee CA, Cho HJ, Hwang SG, Kim KE. Toll-like receptor 9 expression and interferon-α secretion upon CpG-ODN stimulation in allergic subjects. Korean J Pediatr. 2009. 52:1015–1020.
Article15. Bousquet J, Vignola AM, Demoly P. Links between rhinitis and asthma. Allergy. 2003. 58:691–706.
Article16. Emanuel IA, Shah SB. Chronic rhinosinusitis: allergy and sinus computed tomography relationships. Otolaryngol Head Neck Surg. 2000. 123:687–691.
Article17. Nguyen LH, Manoukian JJ, Sobol SE, Tewfik TL, Mazer BD, Schloss MD, Taha R, Hamid QA. Similar allergic inflammation in the middle ear and upper airway: evidence linking otitis media with effusion to the united airway concept. J Allergy Clin Immunol. 2004. 114:1110–1115.
Article18. Ng TP, Tan WC. Epidemiology of allergic rhinitis and its associated risk factors in Singapore. Int J Epidemiol. 1994. 23:553–558.
Article19. Rha YH. Allergic rhinitis in children: diagnosis and treatment. Korean J Pediatr. 2006. 49:593–601.
Article20. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canoncia GW, van Weel C, Agache I, Ait-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O'Heir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wicjman M, Yawn BP, Yorgandipglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chang-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, William D. World Health Organization. GA(2)LEN. AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008. 63:8–160.21. Moscato G, Vandenplas O, Van Wijk RG, Malo JL, Perfetti L, Quirce S, Walusiak J, Castano R, Pala G, Gautrin D, De Groot H, Folletti I, Yacoub MR, Siracusa A. European Academy of Allergology and Clinical Immunology. EAACI position paper on occupational rhinitis. Respir Res. 2009. 10:16.
Article22. Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010. 81:1440–1446.23. Simons FER. H1-receptor antagonist: safety issues. Ann Allergy Asthma Immunol. 1999. 83:481–488.24. Demoly P, Piette V, Daures JP. Treatment of allergic rhinitis during pregnancy. Drugs. 2003. 63:1813–1820.
Article25. Berger WE, White MV. Efficacy of azelastine nasal spray in patients with an unsatisfactory response to loratadine. Ann Allergy Asthma Immunol. 2003. 91:205–211.
Article26. Miadonna A, Tedeschi A, Leggieri E, Lorini M, Folco G, Sala A, Qualizza R, Froldi M, Zanussi C. Behavior and clinical relevance of histamine and leukotrienes C4 and B4 in grass pollen-induced rhinitis. Am Rev Respir Dis. 1987. 136:357–362.
Article27. Kurowski M, Kuna P, Gorski P. Montelukast plus cetrizine in the prophylactic treatment of seasonal allergic rhinitis: influence on clinical symptoms and nasal allergic inflammation. Allergy. 2004. 59:280–288.
Article28. Howarth PH. Leukotrienes in rhinitis. Am J Respir Crit Care Med. 2000. 161:S133–S136.
Article29. Pipkorn U, Proud D, Lichtenstein LM, Kagey-Sobotka A, Norman PS, Naclerio RM. Inhibition of mediator release in allergic rhinitis by pretreatment with topical glucocorticosteroids. N Engl J Med. 1987. 316:1506–1510.
Article30. Pipkorn U. Effect of topical glucocorticoid treatment on nasal mucosal mast cells in allergic rhinitis. Allergy. 1983. 38:125–129.
Article31. Schenkel EJ, Skoner DP, Bronsky EA, Miller SD, Pearlman DS, Rooklin A, Rosen JP, Ruff ME, Vanderwalker ML, Wanderer A, Damaraju CV, Nolop KB, Mesarina-Wicki B. Absence of growth retardation in children with pereneal allergic rhinitis after one year of treatment with mometasone furoate aqueous nasal spray. Pediatrics. 2000. 105:E22.32. Allen DB, Meltzer EO, Lemanske RF Jr, Philpot EE, Faris MA, Kral KM, Prillaman BA, Rickard KA. No growth suppression in children treated with the maximum recommended dose of fluticasone propionate aqueous nasal spray for one year. Allergy Asthma Proc. 2002. 23:407–413.33. Watson WT, Becker AB, Simons FE. Treatment of allergic rhinitis with intranasal corticosteroids in patients with mild asthma: effect on lower airway responsiveness. J Allergy Clin Immunol. 1993. 91:97–101.
Article34. Bousquet J, Demoly P, Michel FB. Specific immunotherapy in rhinitis and asthma. Ann Allergy Asthma Immunol. 2001. 87:38–42.
Article35. Scadding GK, Durham SR, Mirakian R, Jones NS, Leech SC, Farooque S, Ryan D, Walker SM, Clark AT, Dixon TA, Jolles SR, Siddique N, Cullinan P, Howarth PH, Nasser SM. British Society for Allergy and Clinical Immunology. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008. 38:19–42.
Article36. Frew AJ. Sublingual immunotherapy. N Engl J Med. 2008. 358:2259–2264.
Article37. Penagos M, Compalati E, Tarantini F, Baena-Cagnani R, Huerta J, Passalacqua G, Canoncia GW. Efficacy of sublingual immunotherapy in the treatment of allergic rhinitis in pediatric patient 3 to 18 years of age: meta-analysis of randomized, placebo-controlled, double-blind trials. Ann Allergy Asthma Immunol. 2006. 97:141–148.
Article38. Di Rienzo V, Marcucci F, Puccinelli P, Parmiani S, Frati F, Sensi L, Canonica GW, Passalacqua G. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: a 10 year prospective study. Clin Exp Allergy. 2003. 33:206–210.
Article39. Cox LS, Linnemann DL, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006. 117:1021–1035.
Article40. Plewako H, Arvidsson M, Petruson K, Oancea I, Holmberg K, Adelroth E, Gustafsson H, Sandstorm T, Rak S. The effect of omalizumab on nasal allergic inflammation. J Allergy Clin Immunol. 2002. 110:68–71.
Article41. Price KS, Hamilton RG. Anaphylactoid reactions in two patients after omalizumab administration after successful long-term therapy. Allergy Asthma Proc. 2007. 28:313–319.
Article