Diabetes Metab J.
2013 Jun;37(3):196-206. 10.4093/dmj.2013.37.3.196.
Effect of Green Tea Extract/Poly-gamma-Glutamic Acid Complex in Obese Type 2 Diabetic Mice
- Affiliations
-
- 1Department of Physiology, Keimyung University School of Medicine, Daegu, Korea. dksong@kmu.ac.kr
- 2GCB, Co., Ltd., Suwon, Korea.
- 3Department of Pathology, Keimyung University School of Medicine, Daegu, Korea.
- 4Lee Gil Ya Cancer and Diabetes Institute, Gachon University, Incheon, Korea.
- 5Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
- 6Department of Physiology, Yeungnam University College of Medicine, Daegu, Korea. kimyw@yn.med.ac.kr
- KMID: 2174294
- DOI: http://doi.org/10.4093/dmj.2013.37.3.196
Abstract
- BACKGROUND
The increasing prevalence of type 2 diabetes mellitus (T2DM) is associated with the rapid spread of obesity. Obesity induces insulin resistance, resulting in beta-cell dysfunction and thus T2DM. Green tea extract (GTE) has been known to prevent obesity and T2DM, but this effect is still being debated. Our previous results suggested that circulating green tea gallated catechins (GCs) hinders postprandial blood glucose lowering, regardless of reducing glucose and cholesterol absorption when GCs are present in the intestinal lumen. This study aimed to compare the effect of GTE with that of GTE coadministered with poly-gamma-glutamic acid (gamma-PGA), which is likely to inhibit the intestinal absorption of GCs.
METHODS
The db/db mice and age-matched nondiabetic mice were provided with normal chow diet containing GTE (1%), gamma-PGA (0.1%), or GTE+gamma-PGA (1%:0.1%) for 4 weeks.
RESULTS
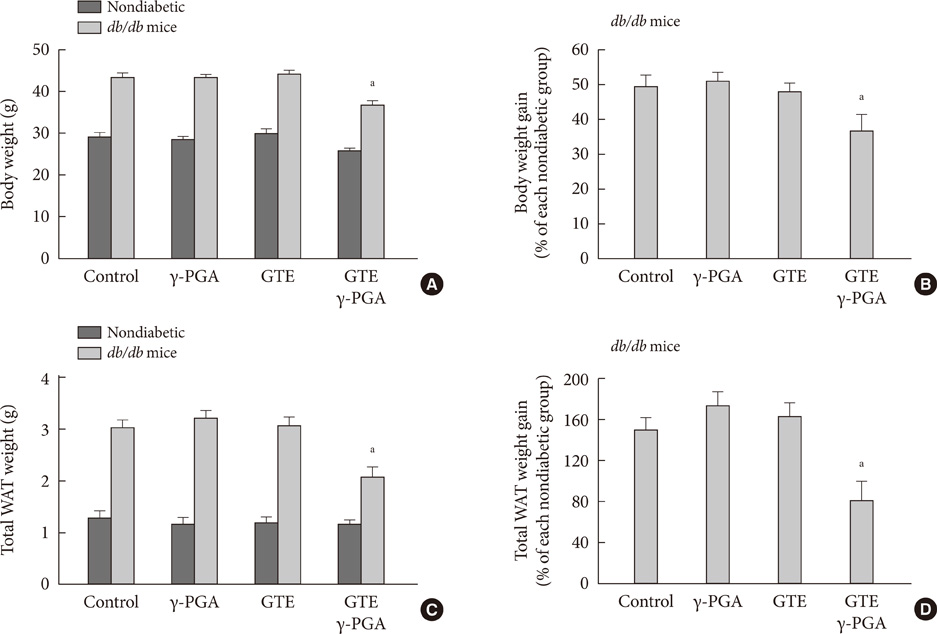
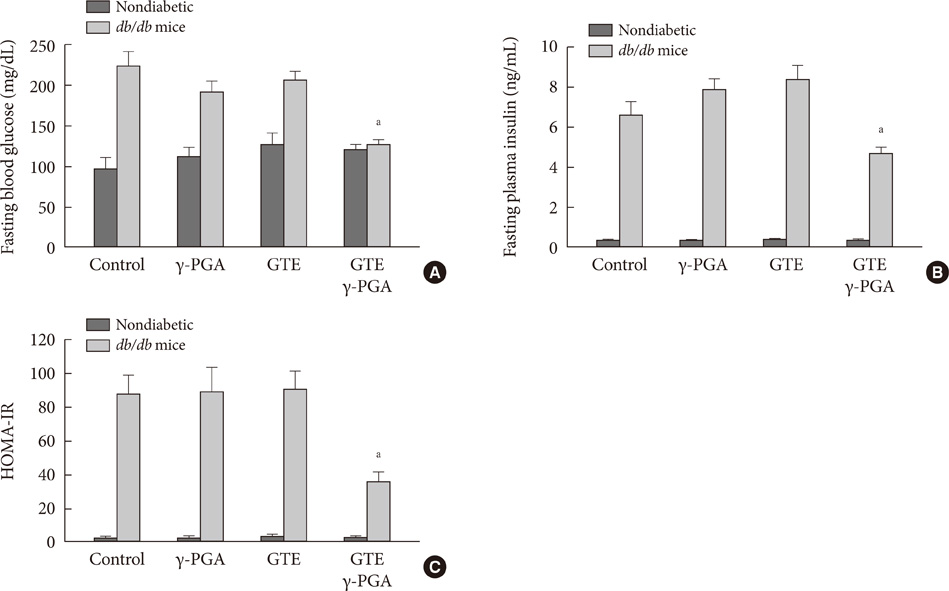
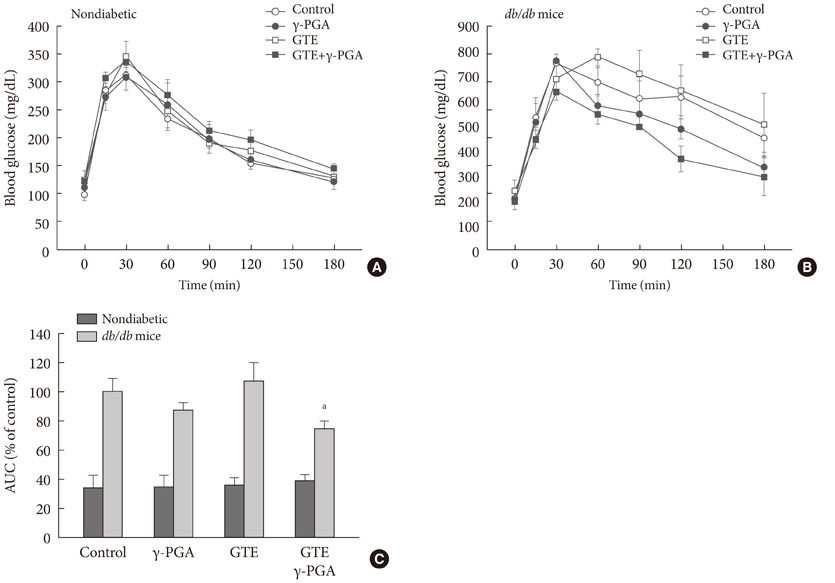
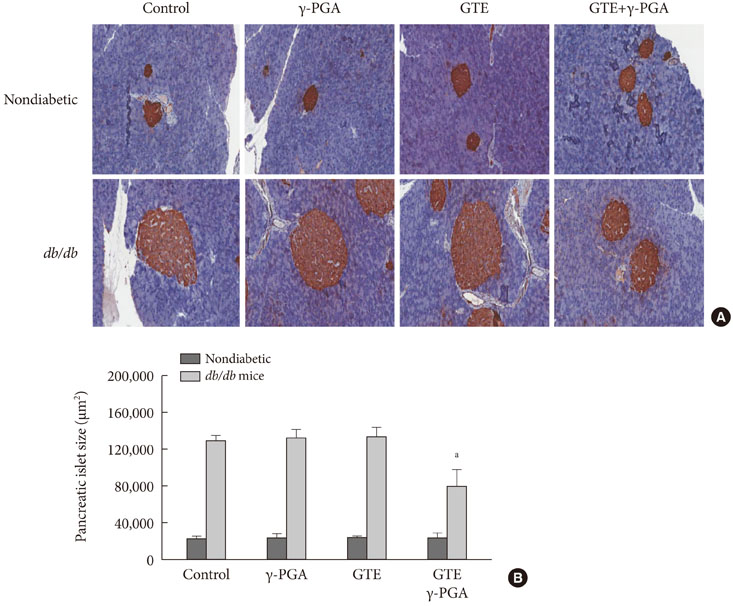
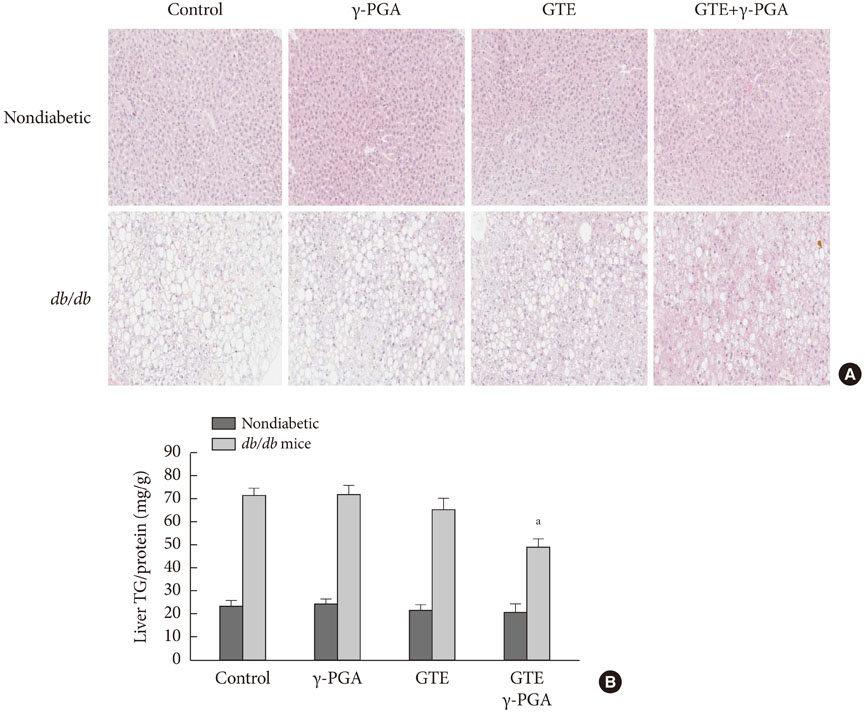
In nondiabetic mice, none of the drugs showed any effects after 4 weeks. In db/db mice, however, weight gain and body fat gain were significantly reduced in the GTE+gamma-PGA group compared to nondrug-treated db/db control mice without the corresponding changes in food intake and appetite. Glucose intolerance was also ameliorated in the GTE+gamma-PGA group. Histopathological analyses showed that GTE+gamma-PGA-treated db/db mice had a significantly reduced incidence of fatty liver and decreased pancreatic islet size. Neither GTE nor gamma-PGA treatment showed any significant results.
CONCLUSION
These results suggest that GTE+gamma-PGA treatment than GTE or gamma-PGA alone may be a useful tool for preventing both obesity and obesity-induced T2DM.
Keyword
MeSH Terms
-
Absorption
Adipose Tissue
Animals
Appetite
Blood Glucose
Catechin
Cholesterol
Diabetes Mellitus, Type 2
Diet
Eating
Fatty Liver
Glucose
Glucose Intolerance
Incidence
Insulin Resistance
Intestinal Absorption
Islets of Langerhans
Mice
Obesity
Polyglutamic Acid
Prevalence
Tea
Weight Gain
Blood Glucose
Catechin
Cholesterol
Glucose
Polyglutamic Acid
Tea
Figure
Reference
-
1. Rossner S. Obesity: the disease of the twenty-first century. Int J Obes Relat Metab Disord. 2002; 26:Suppl 4. S2–S4.2. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001; 286:1195–1200.3. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993; 42:1663–1672.4. Perley M, Kipnis DM. Plasma insulin responses to glucose and tolbutamide of normal weight and obese diabetic and nondiabetic subjects. Diabetes. 1966; 15:867–874.5. Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988; 81:442–448.6. Kahn SE. Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001; 86:4047–4058.7. Park JH, Jin JY, Baek WK, Park SH, Sung HY, Kim YK, Lee J, Song DK. Ambivalent role of gallated catechins in glucose tolerance in humans: a novel insight into non-absorbable gallated catechin-derived inhibitors of glucose absorption. J Physiol Pharmacol. 2009; 60:101–109.8. Kobayashi Y, Suzuki M, Satsu H, Arai S, Hara Y, Suzuki K, Miyamoto Y, Shimizu M. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem. 2000; 48:5618–5623.9. Mabe K, Yamada M, Oguni I, Takahashi T. In vitro and in vivo activities of tea catechins against Helicobacter pylori. Antimicrob Agents Chemother. 1999; 43:1788–1791.10. Takabayashi F, Harada N, Yamada M, Murohisa B, Oguni I. Inhibitory effect of green tea catechins in combination with sucralfate on Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 2004; 39:61–63.11. Hu R, He C, Liu J, Wu Y, Li J, Feng Z, Huang J, Xi XG, Wu Z. Effects of insulin-mimetic vanadyl-poly(gamma-glutamic acid) complex on diabetic rat model. J Pharm Sci. 2010; 99:3041–3047.12. Karmaker S, Saha TK, Yoshikawa Y, Sakurai H. A Zinc(II)/poly(gamma-glutamic acid) complex as an oral therapeutic for the treatment of type-2 diabetic KKAy mice. Macromol Biosci. 2009; 9:279–286.13. Karmaker S, Saha TK, Yoshikawa Y, Sakurai H. Amelioration of hyperglycemia and metabolic syndromes in type 2 diabetic KKA(y) mice by poly (gamma-glutamic acid) oxovanadium (IV) complex. ChemMedChem. 2007; 2:1607–1612.14. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000; 404:661–671.15. Harrold JA. Hypothalamic control of energy balance. Curr Drug Targets. 2004; 5:207–219.16. Johnston K, Sharp P, Clifford M, Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett. 2005; 579:1653–1657.17. Van Amelsvoort JM, Van Hof KH, Mathot JN, Mulder TP, Wiersma A, Tijburg LB. Plasma concentrations of individual tea catechins after a single oral dose in humans. Xenobiotica. 2001; 31:891–901.18. Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004; 4:18.19. Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000; 141:980–987.20. Naftalin RJ, Afzal I, Cunningham P, Halai M, Ross C, Salleh N, Milligan SR. Interactions of androgens, green tea catechins and the antiandrogen flutamide with the external glucose-binding site of the human erythrocyte glucose transporter GLUT1. Br J Pharmacol. 2003; 140:487–499.21. Kim W, Choi K, Kim Y, Park H, Choi J, Lee Y, Oh H, Kwon I, Lee S. Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. strain CK 11-4 screened from Chungkook-Jang. Appl Environ Microbiol. 1996; 62:2482–2488.22. Kwak CS, Lee MS, Park SC. Higher antioxidant properties of Chungkookjang, a fermented soybean paste, may be due to increased aglycone and malonylglycoside isoflavone during fermentation. Nutr Res. 2007; 27:719–727.23. Kwon DY, Jang JS, Lee JE, Kim YS, Shin DH, Park S. The isoflavonoid aglycone-rich fractions of Chungkookjang, fermented unsalted soybeans, enhance insulin signaling and peroxisome proliferator-activated receptor-gamma activity in vitro. Biofactors. 2006; 26:245–258.24. Kwon DY, Jang JS, Hong SM, Lee JE, Sung SR, Park HR, Park S. Long-term consumption of fermented soybean-derived Chungkookjang enhances insulinotropic action unlike soybeans in 90% pancreatectomized diabetic rats. Eur J Nutr. 2007; 46:44–52.25. Sung MH, Park C, Kim CJ, Poo H, Soda K, Ashiuchi M. Natural and edible biopolymer poly-gamma-glutamic acid: synthesis, production, and applications. Chem Rec. 2005; 5:352–366.26. Park JH, Choi JC, Sung MH, Kang JH, Chang MJ. High molecular weight poly-gamma-glutamic acid regulates lipid metabolism in rats fed a high-fat diet and humans. J Microbiol Biotechnol. 2011; 21:766–775.27. Casscells W, Naghavi M, Willerson JT. Vulnerable atherosclerotic plaque: a multifocal disease. Circulation. 2003; 107:2072–2075.28. Lee H, Chang MJ, Kim SH. Effects of poly-gamma-glutamic acid on serum and brain concentrations of glutamate and GABA in diet-induced obese rats. Nutr Res Pract. 2010; 4:23–29.29. Negishi M, Shimomura K, Proks P, Shimomura Y, Mori M. Alpha glucosidase inhibitor voglibose can prevent pioglitazone-induced body weight gain in type 2 diabetic patients. Br J Clin Pharmacol. 2008; 66:318–319.30. Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. 2003; 14:326–332.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of poly-gamma-glutamic acid on serum and brain concentrations of glutamate and GABA in diet-induced obese rats
- Effect of Drinking Green Tea on Glycation and Oxidation of Aortic Collagen in Diabetic Rat
- Effects of Green Tea on Weight Gain, Plasma and Liver Lipids and Lipid Peroxidation in Pair Fed Rats
- The Effects of Poly-gamma Glutamic Acid on Broad Band Ultraviolet B-induced Skin Tumors in a Mice Model
- Antimicrobial effects of green tea extract-containing dentifrice