Blood Res.
2015 Sep;50(3):140-146. 10.5045/br.2015.50.3.140.
Hepatic veno-occlusive disease may develop in secondary iron overloaded mice after allogeneic hematopoietic stem cell transplantation with total body irradiation
- Affiliations
-
- 1Department of Clinical Research Laboratory, Incheon St. Mary's Hospital, Incheon, Korea.
- 2Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea. dcjeong@catholic.ac.kr
- 4Department of Radiation Oncology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Vaccine Bio Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2172756
- DOI: http://doi.org/10.5045/br.2015.50.3.140
Abstract
- BACKGROUND
The outcome of hematopoietic stem cell transplantation (HSCT) is poor in patients with secondary iron overload (SIO). We evaluated the relationship between SIO and veno-occlusive disease (VOD) in an animal model with radiation for HSCT.
METHODS
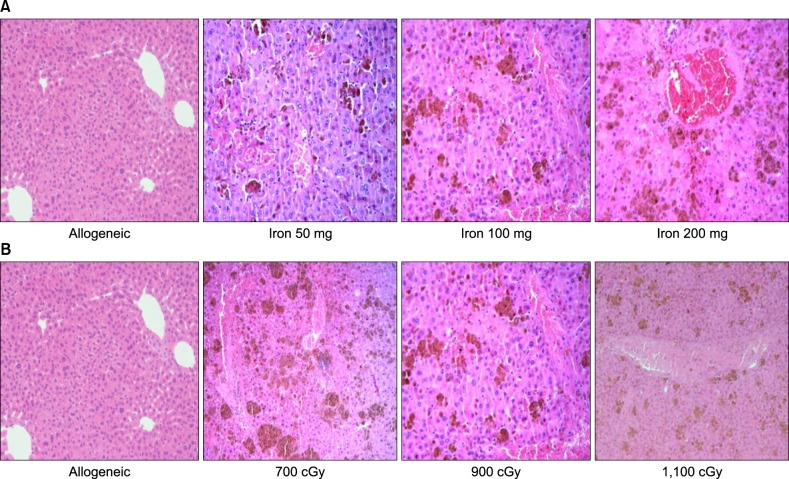
We used a 6-week-old female BDF1 (H-2b/d) and a male C57/BL6 (H-2b) as recipient and donor, respectively. Recipient mice were injected intraperitoneally with 10 mg of iron dextran (cumulative doses of 50 mg, 100 mg, and 200 mg). All mice received total body irradiation for HSCT. We obtained peripheral blood for alanine transaminase (ALT) and liver for pathologic findings, lipid hyperoxide (LH) as reactive oxygen species (ROS), and liver iron content (LIC) on post-HSCT day 1 and day 7. The VOD score was assessed by pathologic findings.
RESULTS
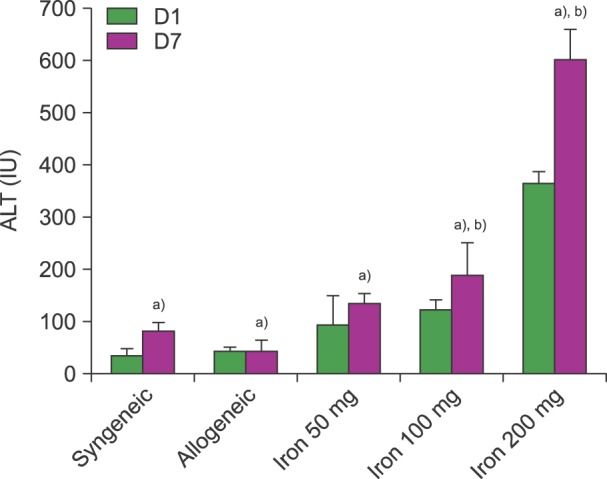
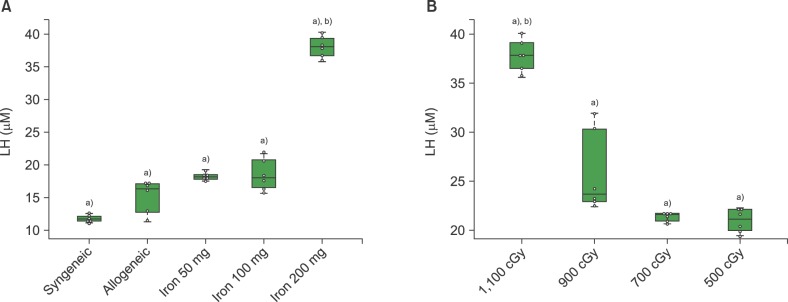
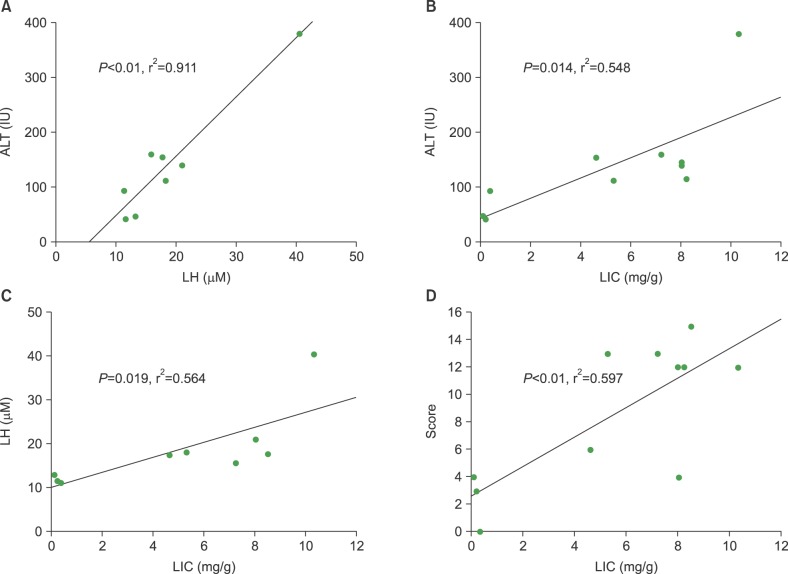
ALT levels increased depending on cumulative iron dose, with significant differences between days 1 and 7 for mice loaded with 200 mg of iron (P<0.01). LH levels significantly increased in mice loaded with 200 mg of iron compared to those in other groups (P<0.01). For mice loaded with 100 mg of iron, the LH level depended on the radiation dose (P<0.01). There was a statistically significant relationship among ALT, LH, and LIC parameters (P<0.05). Pathologic scores for VOD correlated with LIC (P<0.01).
CONCLUSION
Livers with SIO showed high ROS levels depending on cumulative iron dose, and correlations with elevated liver enzyme and LIC. The pathologic score for VOD was associated with the LIC. Our results suggest that SIO may induce VOD after HSCT with irradiation.
MeSH Terms
-
Alanine Transaminase
Animals
Dextrans
Female
Hematopoietic Stem Cell Transplantation*
Hematopoietic Stem Cells*
Hepatic Veno-Occlusive Disease*
Humans
Iron Overload*
Iron*
Liver
Male
Mice*
Models, Animal
Reactive Oxygen Species
Tissue Donors
Whole-Body Irradiation
Alanine Transaminase
Dextrans
Iron
Reactive Oxygen Species
Figure
Cited by 1 articles
-
Biomarkers for hepatic sinusoidal obstruction syndrome after hematopoietic cell transplantation
Je-Hwan Lee
Blood Res. 2015;50(3):123-125. doi: 10.5045/br.2015.50.3.123.
Reference
-
1. Shander A, Cappellini MD, Goodnough LT. Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sang. 2009; 97:185–197. PMID: 19663936.
Article2. Porter JB, Garbowski M. The pathophysiology of transfusional iron overload. Hematol Oncol Clin North Am. 2014; 28:683–701. PMID: 25064708.
Article3. Shander A, Sazama K. Clinical consequences of iron overload from chronic red blood cell transfusions, its diagnosis, and its management by chelation therapy. Transfusion. 2010; 50:1144–1155. PMID: 20088842.
Article4. Jensen PD. Evaluation of iron overload. Br J Haematol. 2004; 124:697–711. PMID: 15009057.
Article5. Jastaniah W, Harmatz P, Pakbaz Z, Fischer R, Vichinsky E, Walters MC. Transfusional iron burden and liver toxicity after bone marrow transplantation for acute myelogenous leukemia and hemoglobinopathies. Pediatr Blood Cancer. 2008; 50:319–324. PMID: 17557314.
Article6. Deeg HJ, Spaulding E, Shulman HM. Iron overload, hematopoietic cell transplantation, and graft-versus-host disease. Leuk Lymphoma. 2009; 50:1566–1572. PMID: 19863335.7. Kanda J, Kawabata H, Chao NJ. Iron overload and allogeneic hematopoietic stem-cell transplantation. Expert Rev Hematol. 2011; 4:71–80. PMID: 21322780.
Article8. Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant. 2008; 41:997–1003. PMID: 18438425.
Article9. Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol. 2014; 4:332–346. PMID: 25755580.
Article10. Lee SH, Yoo KH, Sung KW, et al. Hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Bone Marrow Transplant. 2010; 45:1287–1293. PMID: 20010866.
Article11. Coppell JA, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010; 16:157–168. PMID: 19766729.
Article12. Wadleigh M, Ho V, Momtaz P, Richardson P. Hepatic veno-occlusive disease: pathogenesis, diagnosis and treatment. Curr Opin Hematol. 2003; 10:451–462. PMID: 14564177.
Article13. McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993; 118:255–267. PMID: 8420443.
Article14. Helmy A. Review article: updates in the pathogenesis and therapy of hepatic sinusoidal obstruction syndrome. Aliment Pharmacol Ther. 2006; 23:11–25. PMID: 16393276.
Article15. Ramm GA, Ruddell RG. Hepatotoxicity of iron overload: mechanisms of iron-induced hepatic fibrogenesis. Semin Liver Dis. 2005; 25:433–449. PMID: 16315137.
Article16. Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol. 2011; 21:256–263. PMID: 21939854.
Article17. Moon SN, Han JW, Hwang HS, et al. Establishment of secondary iron overloaded mouse model: evaluation of cardiac function and analysis according to iron concentration. Pediatr Cardiol. 2011; 32:947–952. PMID: 21656238.
Article18. DeLeve LD, McCuskey RS, Wang X, et al. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology. 1999; 29:1779–1791. PMID: 10347121.
Article19. Liu D, He H, Yin D, et al. Mechanism of chronic dietary iron overload-induced liver damage in mice. Mol Med Rep. 2013; 7:1173–1179. PMID: 23404080.
Article20. Fujita N, Miyachi H, Tanaka H, et al. Iron overload is associated with hepatic oxidative damage to DNA in nonalcoholic steatohepatitis. Cancer Epidemiol Biomarkers Prev. 2009; 18:424–432. PMID: 19190144.
Article21. Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation Hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965; 93:200–208.22. Qi K, Li H, An L, et al. The correlation between platelet activation and liver injury by conditioning and bone marrow transplantation. Transplant Proc. 2014; 46:1523–1530. PMID: 24935324.
Article23. Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009; 16:130–143. PMID: 19149566.
Article24. McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology. 2010; 51:1450–1460. PMID: 20373370.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatic veno-occlusive disease resulting in tacrolimus toxicity after allogeneic hematopoietic stem cell transplantation
- Prophylactic Low-dose Heparin or Prostaglandin E1 may Prevent Severe Veno-occlusive Disease of the Liver after Allogeneic Hematopoietic Stem Cell Transplantation in Korean Children
- A Trial Use of Prophylactic Low-Dose Lipo PGE1 (Eglandin) for the Prevention of Hepatic Veno-Occlusive Disease after Hematopoietic Stem Cell Transplantation in Children with Hematologic Malignancies
- Biomarkers for hepatic sinusoidal obstruction syndrome after hematopoietic cell transplantation
- A case of pneumomediastinum combined with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation