Blood Res.
2013 Mar;48(1):55-57. 10.5045/br.2013.48.1.55.
Hepatic veno-occlusive disease resulting in tacrolimus toxicity after allogeneic hematopoietic stem cell transplantation
- Affiliations
-
- 1Catholic Blood and Marrow Transplantation Center, College of Medicine, The Catholic University of Korea, Seoul, Korea. yoojink@catholic.ac.kr
- KMID: 2270728
- DOI: http://doi.org/10.5045/br.2013.48.1.55
Abstract
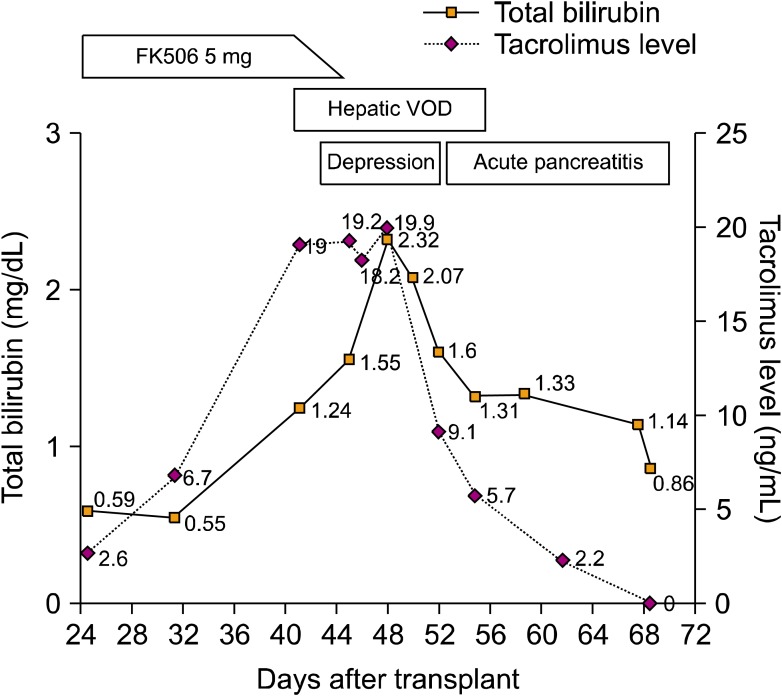
- Tacrolimus is a widely used immunosuppressive agent for the prophylaxis of graft-versus-host disease in allogeneic hematopoietic stem cell transplantation (HSCT). Since tacrolimus is primarily metabolized by the liver, hepatic dysfunction may affect its metabolism. Hepatic veno-occlusive disease (VOD) is an early complication of HSCT that results in hepatic dysfunction, suggesting that VOD may affect tacrolimus metabolism. We report a case of hepatic VOD accompanied by a sustained high blood trough level of tacrolimus despite its discontinuation. The findings of this case suggest that the elimination of tacrolimus can be markedly delayed in patients with hepatic VOD, and that the clinician should carefully modulate the drug dosage for these patients.
MeSH Terms
Figure
Reference
-
1. Przepiorka D, Devine S, Fay J, Uberti J, Wingard J. Practical considerations in the use of tacrolimus for allogeneic marrow transplantation. Bone Marrow Transplant. 1999; 24:1053–1056. PMID: 10578154.
Article2. Iwasaki K. Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics. Drug Metab Pharmacokinet. 2007; 22:328–335. PMID: 17965516.
Article3. Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998; 92:2303–2314. PMID: 9746768.4. Kumar S, DeLeve LD, Kamath PS, Tefferi A. Hepatic venoocclusive disease (sinusoidal obstruction syndrome) after hematopoietic stem cell transplantation. Mayo Clin Proc. 2003; 78:589–598. PMID: 12744547.
Article5. Mori T, Shimizu T, Yamazaki R, Nakazato T, Ikeda Y, Okamoto S. Altered metabolism of tacrolimus in hepatic veno-occlusive disease. Transpl Int. 2005; 18:1215–1217. PMID: 16162110.
Article6. Jain AB, Venkataramanan R, Cadoff E, et al. Effect of hepatic dysfunction and T tube clamping on FK 506 pharmacokinetics and trough concentrations. Transplant Proc. 1990; 22:57–59. PMID: 1689900.7. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004; 43:623–653. PMID: 15244495.
Article8. Shulman HM, Gown AM, Nugent DJ. Hepatic veno-occlusive disease after bone marrow transplantation. Immunohistochemical identification of the material within occluded central venules. Am J Pathol. 1987; 127:549–558. PMID: 2438942.9. McLean AJ, Morgan DJ. Clinical pharmacokinetics in patients with liver disease. Clin Pharmacokinet. 1991; 21:42–69. PMID: 1914341.
Article10. Boswell GW, Bekersky I, Fay J, et al. Tacrolimus pharmacokinetics in BMT patients. Bone Marrow Transplant. 1998; 21:23–28. PMID: 9486490.
Article11. Jusko WJ, Piekoszewski W, Klintmalm GB, et al. Pharmacokinetics of tacrolimus in liver transplant patients. Clin Pharmacol Ther. 1995; 57:281–290. PMID: 7535213.
Article12. Nieto Y, Russ P, Everson G, et al. Acute pancreatitis during immunosuppression with tacrolimus following an allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 2000; 26:109–111. PMID: 10918414.
Article13. Eidelman BH, Abu-Elmagd K, Wilson J, et al. Neurologic complications of FK 506. Transplant Proc. 1991; 23:3175–3178. PMID: 1721398.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Trial Use of Prophylactic Low-Dose Lipo PGE1 (Eglandin) for the Prevention of Hepatic Veno-Occlusive Disease after Hematopoietic Stem Cell Transplantation in Children with Hematologic Malignancies
- Prophylactic Low-dose Heparin or Prostaglandin E1 may Prevent Severe Veno-occlusive Disease of the Liver after Allogeneic Hematopoietic Stem Cell Transplantation in Korean Children
- Biomarkers for hepatic sinusoidal obstruction syndrome after hematopoietic cell transplantation
- A case of pneumomediastinum combined with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation
- Severe Hepatic Sinusoidal Obstruction Syndrome in a Child Receiving Vincristine, Actinomycin-D, and Cyclophosphamide for Rhabdomyosarcoma: Successful Treatment with Defibrotide