Ann Dermatol.
2015 Aug;27(4):364-370. 10.5021/ad.2015.27.4.364.
Protease-Activated Receptor-2 Is Associated with Terminal Differentiation of Epidermis and Eccrine Sweat Glands
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chungnam National University School of Medicine, Daejeon, Korea.
- 2Department of Anatomy, Chungnam National University School of Medicine, Daejeon, Korea. yhlee@cnu.ac.kr
- 3Department of Dermatology, Chungnam National University School of Medicine, Daejeon, Korea.
- 4Department of Physiology, Chungnam National University School of Medicine, Daejeon, Korea.
- 5Seoul Neurology Clinic, Nonsan, Korea.
- 6Department of Plastic Surgery, Konyang University Hospital, Daejeon, Korea.
- KMID: 2171490
- DOI: http://doi.org/10.5021/ad.2015.27.4.364
Abstract
- BACKGROUND
Protease-activated receptor 2 (PAR-2) participates in various biological activities, including the regulation of epidermal barrier homeostasis, inflammation, pain perception, and melanosome transfer in the skin.
OBJECTIVE
To evaluate the basic physiological role of PAR-2 in skin.
METHODS
We investigated PAR-2 expression in human epidermis, skin tumors, and cultured epidermal cells using western blot and immunohistochemical analysis. Additionally, we examined the effect of the PAR-2 agonist, SLIGRL-NH2, on cultured keratinocytes.
RESULTS
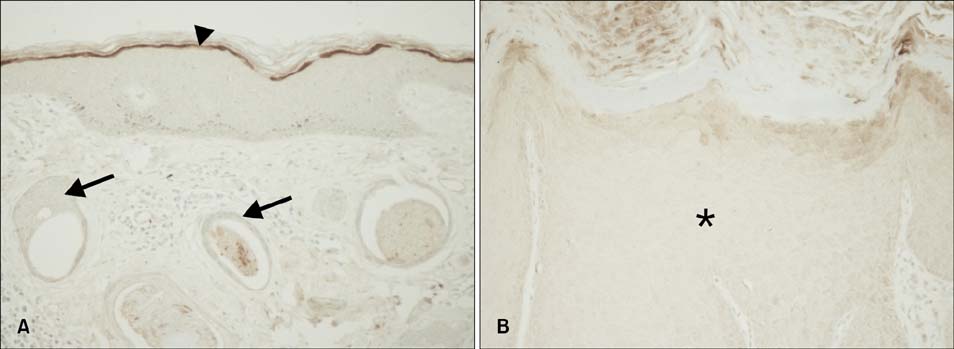
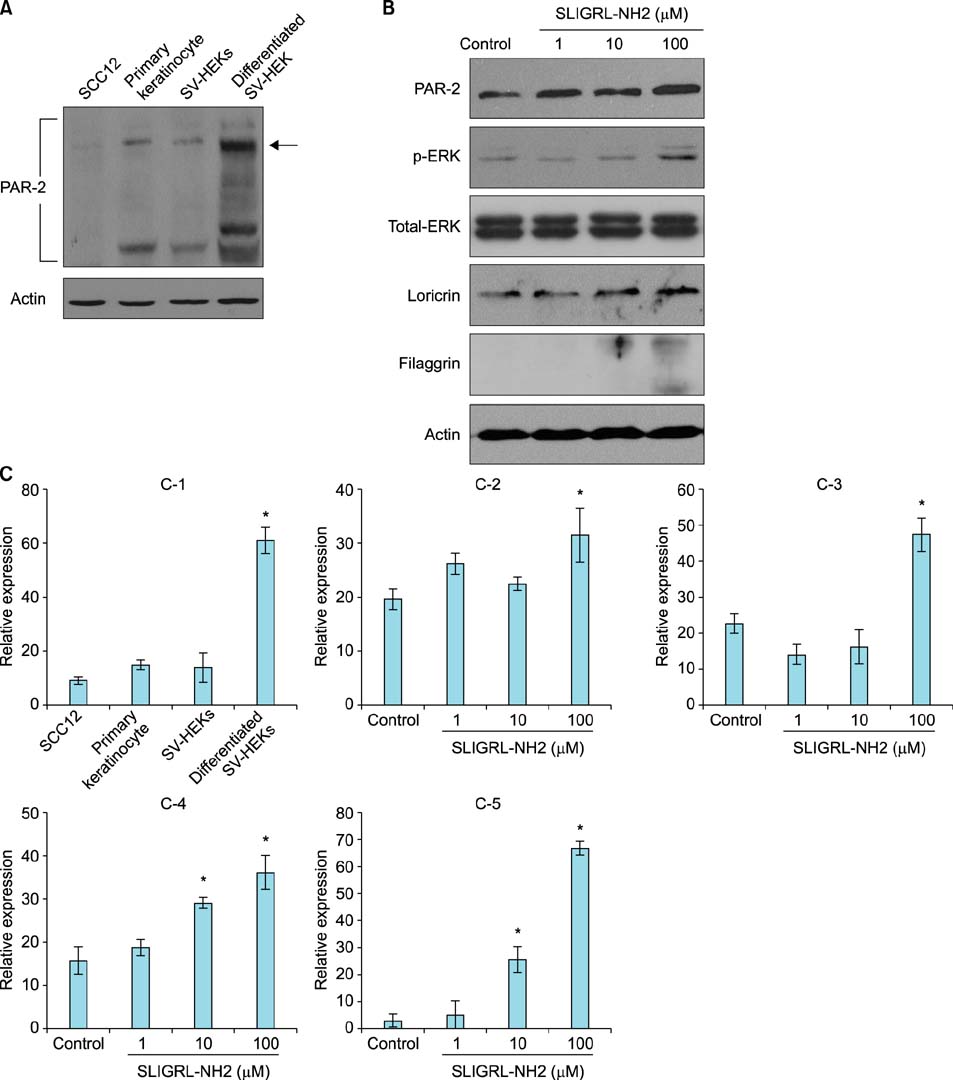
Strong PAR-2 immunoreactivity was observed in the granular layer of normal human skin and the acrosyringium of the eccrine sweat glands. In contrast, weak PAR-2 immunoreactivity was seen in the granular layer of callused skin and in the duct and gland cells of the eccrine sweat glands. Interestingly, PAR-2 immunoreactivity was very weak or absent in the tumor cells of squamous cell carcinoma (SCC) and syringoma. PAR-2 was detected in primary keratinocytes and SV-40T-transformed human epidermal keratinocytes (SV-HEKs), an immortalized keratinocyte cell line, but not in SCC12 cells. SV-HEKs that were fully differentiated following calcium treatment displayed higher PAR-2 expression than undifferentiated SV-HEKs. Treatment of cultured SV-HEKs with PAR-2 agonist increased loricrin and filaggrin expression, a terminal differentiation marker.
CONCLUSION
Our data suggest that PAR-2 is associated with terminal differentiation of epidermis and eccrine sweat glands.
MeSH Terms
Figure
Cited by 1 articles
-
Protease-Activated Receptor-2: A Multifaceted Molecular Transducer in the Human Skin
Hjalte H. Andersen
Ann Dermatol. 2016;28(6):771-772. doi: 10.5021/ad.2016.28.6.771.
Reference
-
1. Demerjian M, Hachem JP, Tschachler E, Denecker G, Declercq W, Vandenabeele P, et al. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol. 2008; 172:86–97.
Article2. Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, et al. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J Invest Dermatol. 2008; 128:1930–1939.
Article3. Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005; 125:510–520.
Article4. Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Invest Dermatol. 2007; 127:1574–1576.
Article5. Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007; 13:975–980.
Article6. Seeliger S, Derian CK, Vergnolle N, Bunnett NW, Nawroth R, Schmelz M, et al. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J. 2003; 17:1871–1885.
Article7. Kim JY, Kim do Y, Son H, Kim YJ, Oh SH. Protease-activated receptor-2 activates NQO-1 via Nrf2 stabilization in keratinocytes. J Dermatol Sci. 2014; 74:48–55.
Article8. Scott G, Deng A, Rodriguez-Burford C, Seiberg M, Han R, Babiarz L, et al. Protease-activated receptor 2, a receptor involved in melanosome transfer, is upregulated in human skin by ultraviolet irradiation. J Invest Dermatol. 2001; 117:1412–1420.
Article9. Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007; 578:715–733.
Article10. Ding-Pfennigdorff D, Averbeck B, Michaelis M. Stimulation of PAR-2 excites and sensitizes rat cutaneous C-nociceptors to heat. Neuroreport. 2004; 15:2071–2075.
Article11. Ando H, Niki Y, Ito M, Akiyama K, Matsui MS, Yarosh DB, et al. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol. 2012; 132:1222–1229.
Article12. Ando H, Niki Y, Yoshida M, Ito M, Akiyama K, Kim JH, et al. Keratinocytes in culture accumulate phagocytosed melanosomes in the perinuclear area. Pigment Cell Melanoma Res. 2010; 23:129–133.
Article13. Rattenholl A, Steinhoff M. Proteinase-activated receptor-2 in the skin: receptor expression, activation and function during health and disease. Drug News Perspect. 2008; 21:369–381.
Article14. Steinhoff M, Corvera CU, Thoma MS, Kong W, McAlpine BE, Caughey GH, et al. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999; 8:282–294.
Article15. Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999; 31:5–19.
Article16. Komatsu N, Saijoh K, Toyama T, Ohka R, Otsuki N, Hussack G, et al. Multiple tissue kallikrein mRNA and protein expression in normal skin and skin diseases. Br J Dermatol. 2005; 153:274–281.
Article17. Kishibe M, Bando Y, Terayama R, Namikawa K, Takahashi H, Hashimoto Y, et al. Kallikrein 8 is involved in skin desquamation in cooperation with other kallikreins. J Biol Chem. 2007; 282:5834–5841.
Article18. Santulli RJ, Derian CK, Darrow AL, Tomko KA, Eckardt AJ, Seiberg M, et al. Evidence for the presence of a protease-activated receptor distinct from the thrombin receptor in human keratinocytes. Proc Natl Acad Sci U S A. 1995; 92:9151–9155.
Article19. Derian CK, Eckardt AJ, Andrade-Gordon P. Differential regulation of human keratinocyte growth and differentiation by a novel family of protease-activated receptors. Cell Growth Differ. 1997; 8:743–749.20. Bovell DL, Kofler B, Lang R. PAR-2 receptor-induced effects on human eccrine sweat gland cells. J Med Invest. 2009; 56:Suppl. 371–374.
Article21. Bovell DL, Santic R, Kofler B, Hermann A, Wilson D, Corbett A, et al. Activation of chloride secretion via proteinase-activated receptor 2 in a human eccrine sweat gland cell line--NCL-SG3. Exp Dermatol. 2008; 17:505–511.
Article22. Rattenholl A, Seeliger S, Buddenkotte J, Schön M, Schön MP, Ständer S, et al. Proteinase-activated receptor-2 (PAR2): a tumor suppressor in skin carcinogenesis. J Invest Dermatol. 2007; 127:2245–2252.
Article23. Yoon HK, Sohn KC, Lee JS, Kim YJ, Bhak J, Yang JM, et al. Prediction and evaluation of protein-protein interaction in keratinocyte differentiation. Biochem Biophys Res Commun. 2008; 377:662–667.
Article24. Kawachi Y, Fujisawa Y, Furuta J, Nakamura Y, Ishii Y, Otsuka F. Superficial epithelioma with sebaceous differentiation: immunohistochemical study of keratinocyte differentiation markers. Eur J Dermatol. 2011; 21:1016–1017.
Article25. Kim SH, Kim S, Choi HI, Choi YJ, Lee YS, Sohn KC, et al. Callus formation is associated with hyperproliferation and incomplete differentiation of keratinocytes, and increased expression of adhesion molecules. Br J Dermatol. 2010; 163:495–501.
Article26. Jeon GA, Lee JS, Patel V, Gutkind JS, Thorgeirsson SS, Kim EC, et al. Global gene expression profiles of human head and neck squamous carcinoma cell lines. Int J Cancer. 2004; 112:249–258.
Article27. Back SJ, Im M, Sohn KC, Choi DK, Shi G, Jeong NJ, et al. Epigenetic modulation of gene expression during keratinocyte differentiation. Ann Dermatol. 2012; 24:261–266.
Article28. Gazel A, Nijhawan RI, Walsh R, Blumenberg M. Transcriptional profiling defines the roles of ERK and p38 kinases in epidermal keratinocytes. J Cell Physiol. 2008; 215:292–308.
Article