Cancer Res Treat.
2008 Mar;40(1):27-32.
Effect on Cell Cycle Progression by N-Myc Knockdown in SK-N-BE(2) Neuroblastoma Cell Line and Cytotoxicity with STI-571 Compound
- Affiliations
-
- 1Department of Microbiology, Ewha Womans University School of Medicine, Seoul, Korea. soyounwoo@ewha.ac.kr
- 2Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea.
Abstract
-
PURPOSE: Neuroblastoma is a common tumor in childhood, and generally exhibits heterogeneity and a malignant progression. MYCN expression and amplification profiles frequently correlate with therapeutic prognosis. Although it has been reported that MYCN silencing causes differentiation and apoptosis in human neuroblastoma cells, MYCN expression influences the cytotoxic potential of chemotherapeutic drugs via the deregulation of the cell cycle. STI-571 may constitute a promising therapeutic agent against neuroblastoma, particularly in cases in which c-Kit is expressed preferentially in MYCN-amplified neuroblastoma.
MATERIALS AND METHODS
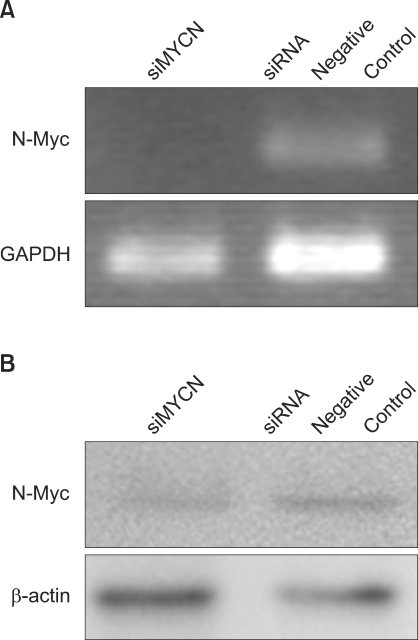
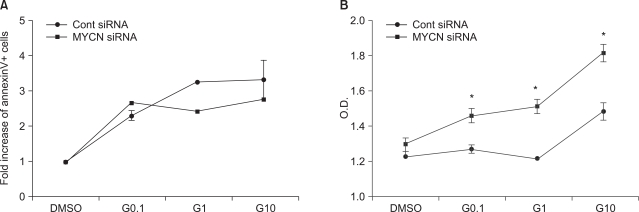
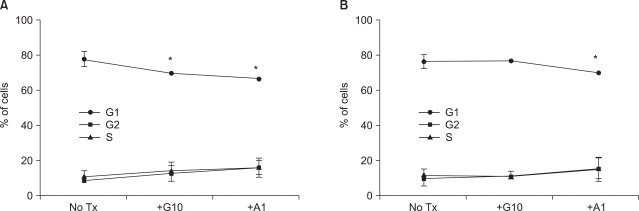
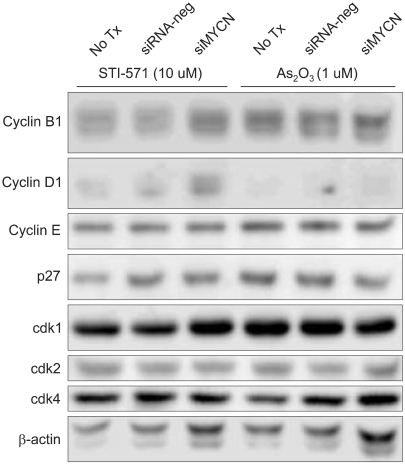
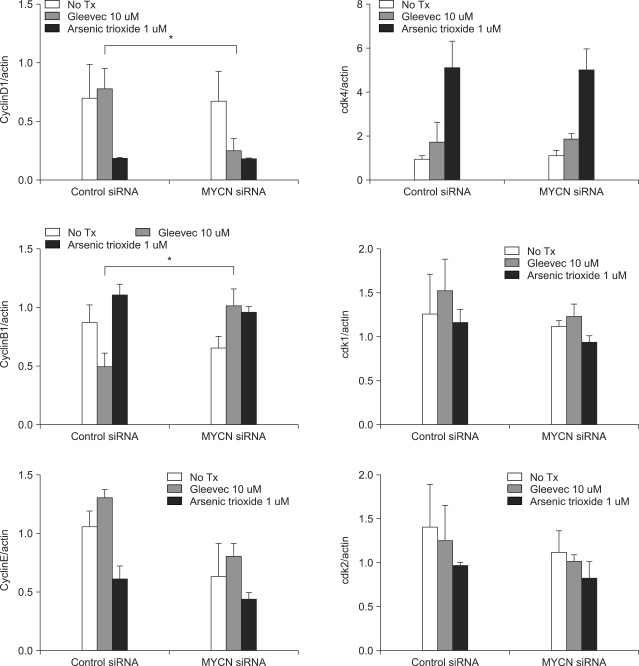
To determine whether STI-571 exerts a synergistic effect on cytotoxicity with MYCN expression, we assessed apoptotic cell death and cell cycle distribution after 72 h of exposure to STI-571 with or with out treatment of SK-N-BE(2) neuroblastoma cells with MYCN siRNA.
RESULTS
MYCN siRNA-treated SK-N-BE(2) cells did not affect apoptosis and cells were arrested in G0/G1 phase after STI-571 treatment.
CONCLUSIONS
siRNA therapy targeted to MYCN may not be effective when administered in combination with STI-571 treatment in cases of neuroblastoma. Therefore, chemotherapeutic drugs that target S or G2-M phase may prove ineffective when applied to cells arrested in the G0/1 phase as the result of MYCN knockdown and STI-571 treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Kang JH, Rychahou PG, Ishola TA, Qiao J, Evers BM, Chung DH. MYCN silencing induces differentiation and apoptosis in human neuroblastoma cells. Biochem Biophys Res Commun. 2006; 351:192–197. PMID: 17055458.
Article2. Paffhausen T, Schwab M, Westermann F. Targeted MYCN expression affects cytotoxic potential of chemotherapeutic drugs in neuroblastoma cells. Cancer Lett. 2007; 250:17–24. PMID: 17141950.
Article3. Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005; 353:172–187. PMID: 16014887.
Article4. Mauro MJ, O'Dwyer M, Heinrich MC, Druker BJ. STI571: a paradigm of new agents for cancer therapeutics. J Clin Oncol. 2002; 20:325–334. PMID: 11773186.
Article5. Vitali R, Cesi V, Nicotra MR, McDowell HP, Donfrancesco A, Mannarino O, et al. c-Kit is preferentially expressed in MYCN-amplified neuroblastoma and its effect on cell proliferation is inhibited in vitro by STI-571. Int J Cancer. 2003; 106:147–152. PMID: 12800187.6. Meyer N, Kim SS, Penn LZ. The Oscar-worthy role of Myc in apoptosis. Semin Cancer Biol. 2006; 16:275–287. PMID: 16945552.
Article7. Schwab M, Alitalo K, Klempnauer KH, Varmus HE, Bishop JM, Gilbert F, et al. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983; 305:245–248. PMID: 6888561.
Article9. Pession A, Tonelli R. The MYCN oncogene as a specific and selective drug target for peripheral and central nervous system tumors. Curr Cancer Drug Targets. 2005; 5:273–283. PMID: 15975048.
Article10. Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000; 295:139–145. PMID: 10991971.11. Gilbert F, Feder M, Balaban G, Brangman D, Lurie DK, Podolsky R, et al. Human neuroblastomas and abnormalities of chromosomes 1 and 17. Cancer Res. 1984; 44:5444–5449. PMID: 6488196.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Etoposide Reduces Peroxynitrite-Induced Cytotoxicity via Direct Scavenging Effect
- The antitumor effect of various cytokines on human neuroblastoma cell lines SK-N-SH, IMR-32, and SK-N-MC
- Antiproliferative and Cytotoxic Effects of Resveratrol in Mitochondria-Mediated Apoptosis in Rat B103 Neuroblastoma Cells
- Effect of BMI1 Knockdown on Cell Proliferation, Apoptosis, Invasiveness, and Migration of U251 Glioma Cells
- Apoptosis and Cell Cycle Arrest in Two Human Breast Cancer Cell Lines by Dieckol Isolated from Ecklonia cava