Int J Stem Cells.
2016 May;9(1):79-89. 10.15283/ijsc.2016.9.1.79.
Study of the Effect of Route of Administration of Mesenchymal Stem Cells on Cisplatin-Induced Acute Kidney Injury in Sprague Dawley Rats
- Affiliations
-
- 1Department of Pathology, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
- 2Urology and Nephrology Center, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
- 3Department of Clinical Pharmacology, Faculty of Medicine, Mansoura University, Mansoura, Egypt. aboelkhairmohamed@yahoo.com
- 4Mansoura Medical Experimental Research Center, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
- KMID: 2164164
- DOI: http://doi.org/10.15283/ijsc.2016.9.1.79
Abstract
- BACKGROUND AND OBJECTIVES
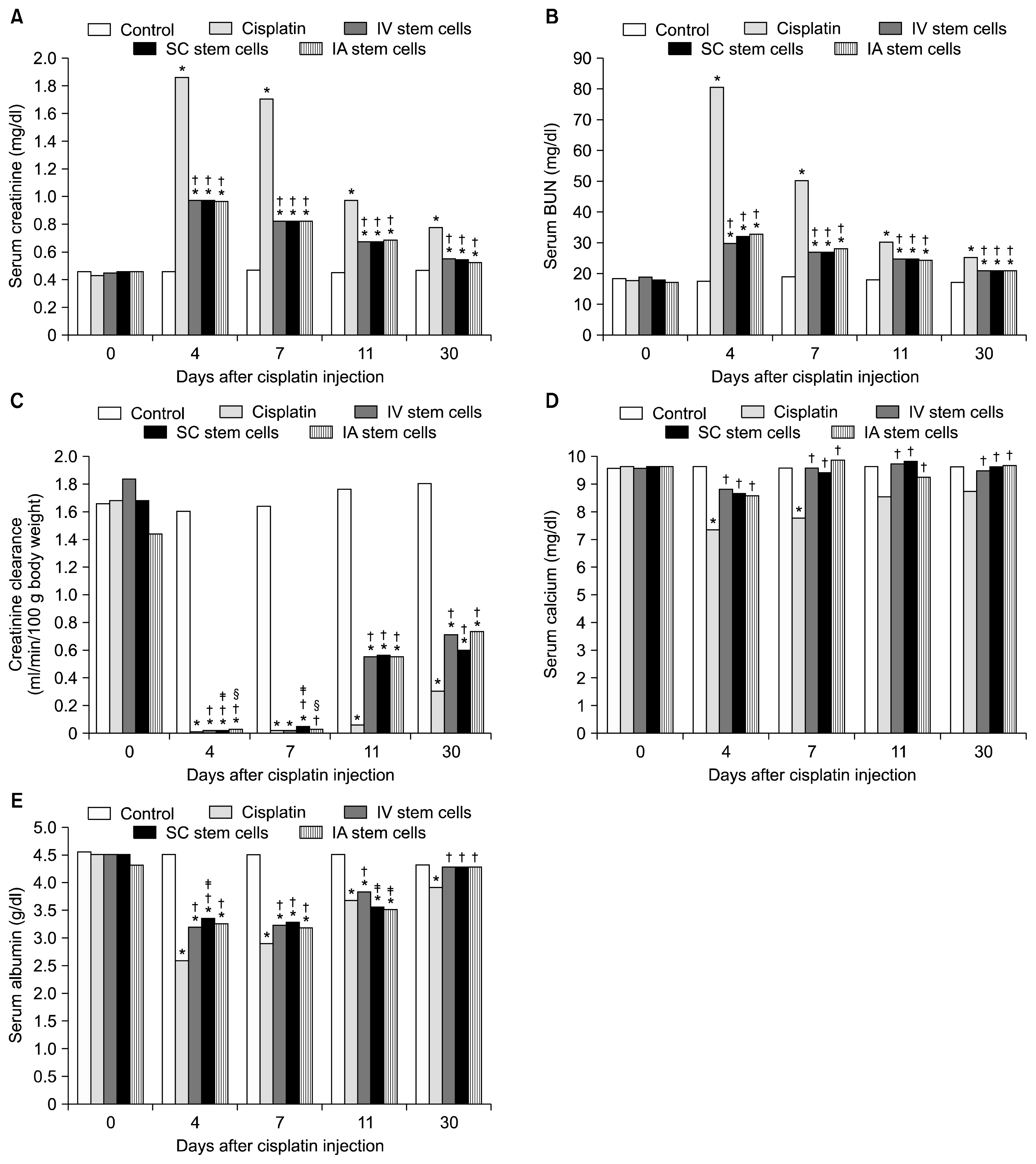
Mesenchymal stem cells (MSCs) have been shown to ameliorate cisplatin-induced acute kidney injury (AKI). The present study compares the efficacy of different routes of MSCs administration on kidney damage and regeneration after cisplatin-induced AKI.
METHODS
A single intraperitoneal injection of cisplatin (5 mg/kg) was used to induce AKI in 160 rats. MSCs (5×106) were given by either intravenous, intra-arterial or kidney sub capsular injection one day after cisplatin injection. Suitable control groups were included. Rats were sacrificed at 4, 7, 11 and 30 days after cisplatin injection. Kidney function parameters, kidney tissue oxidative stress markers, and scoring for renal tissue injury, regeneration and chronicity were all determined.
RESULTS
MSCs by any routes were able to ameliorate kidney function deterioration and renal tissue damage induced by cisplatin. The overall results of the three routes were equal. Differences between the different routes in one parameter were transient and inconsistent with other parameters.
CONCLUSION
Changing the route of MSCs injection does not have a major influence on the outcome. Future evaluation should focus on differences between the routes of administration considering the long term safety.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007; 18:2486–2496. DOI: 10.1681/ASN.2007020140. PMID: 17656474.
Article2. Sahu KM, Mukhiya GK, Begum F, Ahmed T, Ashrafee F, Mutawaqqel Alallah M, Hoque SM, Zayed S. Repair and recovery of acute kidney injury. Clin Query Nephrol. 2012; 1:95–98. DOI: 10.1016/S2211-9477(11)70013-9.
Article3. Erpicum P, Detry O, Weekers L, Bonvoisin C, Lechanteur C, Briquet A, Beguin Y, Krzesinski JM, Jouret F. Mesenchymal stromal cell therapy in conditions of renal ischaemia/reperfusion. Nephrol Dial Transplant. 2014; 29:1487–1493. DOI: 10.1093/ndt/gft538. PMID: 24516234.
Article4. Kim JH, Park DJ, Yun JC, Jung MH, Yeo HD, Kim HJ, Kim DW, Yang JI, Lee GW, Jeong SH, Roh GS, Chang SH. Human adipose tissue-derived mesenchymal stem cells protect kidneys from cisplatin nephrotoxicity in rats. Am J Physiol Renal Physiol. 2012; 302:F1141–F1150. DOI: 10.1152/ajprenal.00060.2011.
Article5. Peng X, Xu H, Zhou Y, Wang B, Yan Y, Zhang X, Wang M, Gao S, Zhu W, Xu W, Qian H. Human umbilical cord mesenchymal stem cells attenuate cisplatin-induced acute and chronic renal injury. Exp Biol Med (Maywood). 2013; 238:960–970. DOI: 10.1177/1477153513497176.
Article6. Tögel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009; 18:475–485. DOI: 10.1089/scd.2008.0092.
Article7. Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, Young RG, Marcelino M, Pittenger MF, Solaiyappan M, Boston RC, Tsui BM, Wahl RL, Bulte JW. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005; 112:1451–1461. DOI: 10.1161/CIRCULATIONAHA.105.537480. PMID: 16129797. PMCID: 1456731.
Article8. Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007; 72:430–441. DOI: 10.1038/sj.ki.5002334. PMID: 17507906.
Article9. Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006; 27:1114–1122. DOI: 10.1093/eurheartj/ehi818. PMID: 16510464.
Article10. Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, Edelstein CL. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther. 2007; 322:8–15. DOI: 10.1124/jpet.107.119792. PMID: 17400889.
Article11. Lu LH, Oh DJ, Dursun B, He Z, Hoke TS, Faubel S, Edelstein CL. Increased macrophage infiltration and frac-talkine expression in cisplatin-induced acute renal failure in mice. J Pharmacol Exp Ther. 2008; 324:111–117. DOI: 10.1124/jpet.107.130161.
Article12. Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, Pittenger MF, van Zijl PC, Huang J, Bulte JW. Dual-modality monitoring of targeted intra-arterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008; 39:1569–1574. DOI: 10.1161/STROKEAHA.107.502047. PMID: 18323495. PMCID: 2857730.
Article13. Pereira RF, Halford KW, O’Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995; 92:4857–4861. DOI: 10.1073/pnas.92.11.4857. PMID: 7761413. PMCID: 41806.
Article14. Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999; 72:570–585. DOI: 10.1002/(SICI)1097-4644(19990315)72:4<570::AID-JCB12>3.0.CO;2-W. PMID: 10022616.
Article15. Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003; 17:160–170. DOI: 10.1038/sj.leu.2402763. PMID: 12529674.
Article16. Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004; 103:1662–1668. DOI: 10.1182/blood-2003-09-3070.
Article17. van Roeyen CR, Ostendorf T, Denecke B, Bokemeyer D, Behrmann I, Strutz F, Lichenstein HS, LaRochelle WJ, Pena CE, Chaudhuri A, Floege J. Biological responses to PDGF-BB versus PDGF-DD in human mesangial cells. Kidney Int. 2006; 69:1393–1402. DOI: 10.1038/sj.ki.5000332. PMID: 16557224.
Article18. Seghatoleslam M, Jalali M, Alamdari DH, Nikravesh MR, Hoseini M, Fazel A, Koliakos G. Optimal incubating time of in vitro bromodeoxyuridine labeling of human umbilical cord blood- mononuclear cells and their functional assessment in ICH rats. J Cell Animal Biology. 2012; 6:144–153.19. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358. DOI: 10.1016/0003-2697(79)90738-3. PMID: 36810.
Article20. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82:70–77. DOI: 10.1016/0003-9861(59)90090-6. PMID: 13650640.
Article21. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008; 73:994–1007. DOI: 10.1038/sj.ki.5002786. PMID: 18272962.
Article22. Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001; 169:12–20. DOI: 10.1159/000047856. PMID: 11340257.
Article23. Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007; 39:573–576. DOI: 10.1016/j.transproceed.2006.12.019. PMID: 17362785.
Article24. Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009; 113:816–826. DOI: 10.1182/blood-2007-12-128702. PMCID: 2630267.
Article25. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009; 5:54–63. DOI: 10.1016/j.stem.2009.05.003. PMID: 19570514. PMCID: 4154377.
Article26. Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003; 101:2999–3001. DOI: 10.1182/blood-2002-06-1830.
Article27. Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008; 2:284–291. DOI: 10.1016/j.stem.2008.01.014. PMID: 18371453.
Article28. Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008; 59:311–325. DOI: 10.1146/annurev.med.59.061506.154239.
Article29. Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007; 18:1754–1764. DOI: 10.1681/ASN.2007010044. PMID: 17460140.
Article30. Esson ML, Schrier RW. Diagnosis and treatment of acute tubular necrosis. Ann Intern Med. 2002; 137:744–752. DOI: 10.7326/0003-4819-137-9-200211050-00010. PMID: 12416948.
Article31. Gill N, Nally JV Jr, Fatica RA. Renal failure secondary to acute tubular necrosis: epidemiology, diagnosis, and management. Chest. 2005; 128:2847–2863. DOI: 10.1378/chest.128.4.2847. PMID: 16236963.32. Nankivell B. Creatinine clearance and the assessment of renal function. Aust Prescr. 2001; 24:15–17. DOI: 10.18773/austprescr.2001.009.
Article33. Jones TW, Chopra S, Kaufman JS, Flamenbaum W, Trump BF. Cis-diamminedichloroplatinum (II)-induced acute renal failure in the rat. Correlation of structural and functional alterations. Lab Invest. 1985; 52:363–374. PMID: 4039014.34. Zhang JG, Lindup WE. Cisplatin-induced nephrotoxicity in vitro: increases in cytosolic calcium concentration and the inhibition of cytosolic and mitochondrial protein kinase C. Toxicol Lett. 1996; 89:11–17. DOI: 10.1016/S0378-4274(96)03776-9. PMID: 8952706.
Article35. Cao H, Qian H, Xu W, Zhu W, Zhang X, Chen Y, Wang M, Yan Y, Xie Y. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett. 2010; 32:725–732. DOI: 10.1007/s10529-010-0207-y. PMID: 20131083.
Article36. Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, Chang KC, Leu S, Yip HK. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011; 9:51. DOI: 10.1186/1479-5876-9-51. PMID: 21545725. PMCID: 3112438.
Article37. Broekema M, Harmsen MC, van Luyn MJ, Koerts JA, Petersen AH, van Kooten TG, van Goor H, Navis G, Popa ER. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol. 2007; 18:165–175. DOI: 10.1681/ASN.2005070730.
Article38. Yamate J, Tatsumi M, Nakatsuji S, Kuwamura M, Kotani T, Sakuma S. Immunohistochemical observations on the kinetics of macrophages and myofibroblasts in rat renal interstitial fibrosis induced by cis-diamminedichloroplatinum. J Comp Pathol. 1995; 112:27–39. DOI: 10.1016/S0021-9975(05)80087-8. PMID: 7536759.
Article39. Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, Miao S, Liu G, Lu M, Zhu Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014; 5:40–53. DOI: 10.1186/scrt428.
Article40. Kinomura M, Kitamura S, Tanabe K, Ichinose K, Hirokoshi K, Takazawa Y, Kitayama H, Nasu T, Sugiyama H, Yamasaki Y, Sugaya T, Maeshima Y, Makino H. Amelioration of cisplatin-induced acute renal injury by renal progenitor-like cells derived from the adult rat kidney. Cell Transplant. 2008; 17:143–158. DOI: 10.3727/000000008783907008. PMID: 18468244.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amniotic Fluid-Derived Mesenchymal Stem Cells Cut Short the Acuteness of Cisplatin-Induced Nephrotoxicity in Sprague-Dawley Rats
- The Effect of Different Routes of Injection of Bone Marrow Mesenchymal Stem Cells on Parotid Glands of Rats Receiving Cisplatin: A Comparative Study
- Effects of Human Adipose-Derived Stem Cells in Regenerating the Damaged Renal Tubular Epithelial Cells in an Animal Model of Cisplatin-Induced Acute Kidney Injury
- Post-treatment Effects of Erythropoietin and Nordihydroguaiaretic Acid on Recovery from Cisplatin-induced Acute Renal Failure in the Rat
- Autophagy localization and cytoprotective role in cisplatin-induced acute kidney injury