J Vet Sci.
2015 Mar;16(1):17-23. 10.4142/jvs.2015.16.1.17.
Protective effect of butylated hydroxylanisole against hydrogen peroxide-induced apoptosis in primary cultured mouse hepatocytes
- Affiliations
-
- 1College of Pharmacy, Research Institute of Pharmaceutical Sciences, Kyungpook National University, Daegu 702-701, Korea. vetmedic@knu.ac.kr
- 2Department of Veterinary Physiology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 3Department of Veterinary Physiology, College of Veterinary Medicine, Chonnam National University, Gwangju 500-757, Korea.
- 4Department of Cardiovascular and Neurologic Disease, College of Oriental Medicine, Daegu Haany University, Daegu 706-828, Korea.
- 5Department of Biology Education, College of Education, Pusan National University, Busan 609-735, Korea.
- 6Research Center, Dongnam Institute of Radiological and Medical Sciences, Busan 619-953, Korea.
- 7Department of Veterinary Anatomy, College of Veterinary Medicine, Chonnam National University, Gwangju 500-757, Korea.
- 8Department of Immunobiology, Yale University School of Medicine, New Haven, CT 06510, USA.
- KMID: 2160816
- DOI: http://doi.org/10.4142/jvs.2015.16.1.17
Abstract
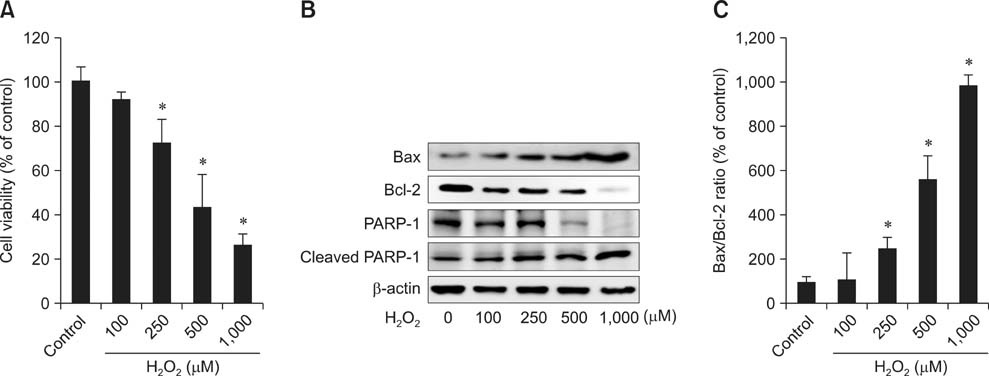
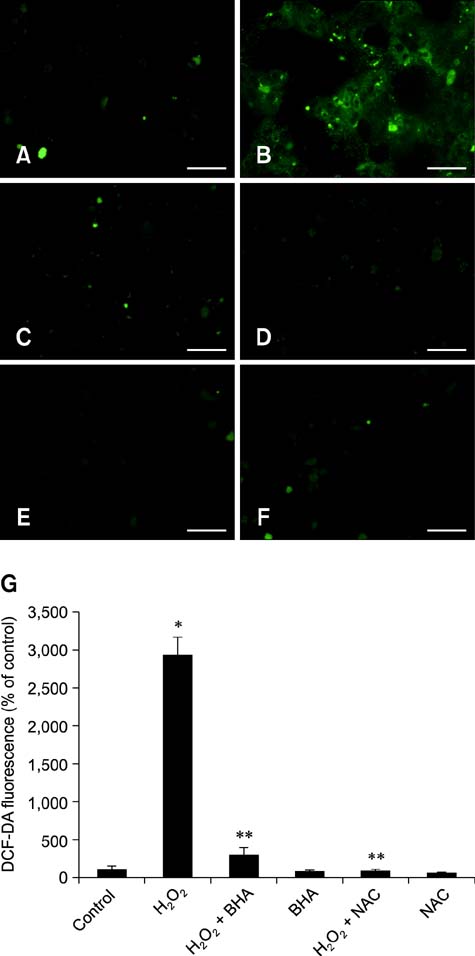
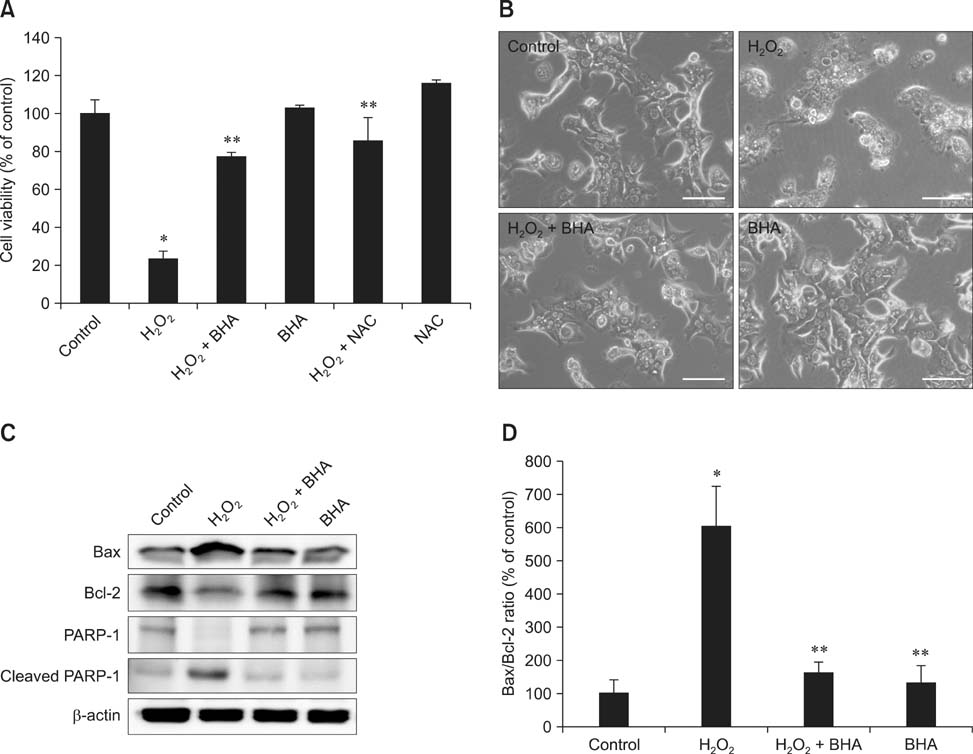
- Butylated hydroxyanisole (BHA) is a synthetic phenolic compound consisting of a mixture of two isomeric organic compounds: 2-tert-butyl-4-hydroxyanisole and 3-tert-butyl-4-hydroxyanisole. We examined the effect of BHA against hydrogen peroxide (H2O2)-induced apoptosis in primary cultured mouse hepatocytes. Cell viability was significantly decreased by H2O2 in a dose-dependent manner. Additionally, H2O2 treatment increased Bax, decreased Bcl-2, and promoted PARP-1 cleavage in a dose-dependent manner. Pretreatment with BHA before exposure to H2O2 significantly attenuated the H2O2-induced decrease of cell viability. H2O2 exposure resulted in an increase of intracellular reactive oxygen species (ROS) generation that was significantly inhibited by pretreatment with BHA or N-acetyl-cysteine (NAC, an ROS scavenger). H2O2-induced decrease of cell viability was also attenuated by pretreatment with BHA and NAC. Furthermore, H2O2-induced increase of Bax, decrease of Bcl-2, and PARP-1 cleavage was also inhibited by BHA. Taken together, results of this investigation demonstrated that BHA protects primary cultured mouse hepatocytes against H2O2-induced apoptosis by inhibiting ROS generation.
MeSH Terms
Figure
Reference
-
1. Aviram M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res. 2000; 33:Suppl. S85–S97.2. Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004; 74:2157–2184.
Article3. Chen KC, Zhou Y, Zhang W, Lou MF. Control of PDGF-induced reactive oxygen species (ROS) generation and signal transduction in human lens epithelial cells. Mol Vis. 2007; 13:374–387.4. Cole KK, Perez-Polo JR. Poly(ADP-ribose) polymerase inhibition prevents both apoptotic-like delayed neuronal death and necrosis after H2O2 injury. J Neurochem. 2002; 82:19–29.
Article5. Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PLM, Moshage H. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2006; 44:918–929.
Article6. Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004; 66:361–384.
Article7. de Magalhães JP, Church GM. Cells discover fire: employing reactive oxygen species in development and consequences for aging. Exp Gerontol. 2006; 41:1–10.8. Dumont A, Hehner SP, Hofmann TG, Ueffing M, Dröge W, Schmitz ML. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-κ B. Oncogene. 1999; 18:747–757.
Article9. Englert RP, Shacter E. Distinct modes of cell death induced by different reactive oxygen species: amino acyl chloramines mediate hypochlorous acid-induced apoptosis. J Biol Chem. 2002; 277:20518–20526.
Article10. Evans ZP, Mandavilli BS, Ellett JD, Rodwell D, Fariss MW, Fiorini RN, Schnellmann RG, Schmidt MG, Chavin K. Vitamin E succinate enhances steatotic liver energy status and prevents oxidative damage following ischemia/reperfusion. Transplant Proc. 2009; 41:4094–4098.
Article11. Fang L, Gou S, Fang X, Cheng L, Fleck C. Current progresses of novel natural products and their derivatives/analogs as anti-Alzheimer candidates: an update. Mini Rev Med Chem. 2013; 13:870–887.
Article12. Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994; 344:721–724.
Article13. Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008; 320:661–664.
Article14. Iverson F. In vivo studies on butylated hydroxyanisole. Food Chem Toxicol. 1999; 37:993–997.15. Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury. J Gastroenterol Hepatol. 2000; 15:718–724.
Article16. Jiang J, Yu S, Jiang Z, Liang C, Yu W, Li J, Du X, Wang H, Gao X, Wang X. N-acetyl-serotonin protects HepG2 cells from oxidative stress injury induced by hydrogen peroxide. Oxid Med Cell Longev. 2014; 2014:310504.
Article17. Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999; 47:3954–3962.
Article18. Kim KC, Lee C. Curcumin induces downregulation of E2F4 expression and apoptotic cell death in HCT116 human colon cancer cells; involvement of reactive oxygen species. Korean J Physiol Pharmacol. 2010; 14:391–397.
Article19. Lee MY, Jung SC, Lee JH, Han HJ. Estradiol-17β protects against hypoxia-induced hepatocyte injury through ER-mediated upregulation of Bcl-2 as well as ER-independent antioxidant effects. Cell Res. 2008; 18:491–499.
Article20. Li WM, Liu HT, Li XY, Wu JY, Xu G, Teng YZ, Ding ST, Yu C. The effect of tetramethylpyrazine on hydrogen peroxide-induced oxidative damage in human umbilical vein endothelial cells. Basic Clin Pharmacol Toxicol. 2010; 106:45–52.
Article21. Loguercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003; 34:1–10.
Article22. Lu Y, Zhang YY, Hu YC, Lu YH. Protective effects of 2',4'-dihydroxy-6'-methoxy-3',5'-dimethylchalcone against hydrogen peroxide-induced oxidative stress in hepatic L02 cell. Arch Pharm Res. 2014; 37:1211–1218.
Article23. Martin D, Salinas M, Fujita N, Tsuruo T, Cuadrado A. Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J Biol Chem. 2002; 277:42943–42952.24. Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007; 26:1101–1109.
Article25. Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Miyazawa T, Ishibashi K, Horie T, Imai K, Todoroki T, Kimura S, Koike K. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res. 2001; 61:4365–4370.26. Murakami Y, Shoji M, Ogiwara T, Tanaka S, Yokoe I, Fujisawa S. Preventive effect of ortho dimer of butylated hydroxyanisole on activator protein-1 activation and cyclooxygenase-2 expression in macrophages stimulated by fimbriae of Porphyromonas gingivalis, an oral anaerobe. Anticancer Res. 2006; 26:2915–2920.27. Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008; 4:89–96.28. Rajendran P, Nandakumar N, Rengarajan T, Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, Gopas J, Nishigaki I. Antioxidants and human diseases. Clin Chim Acta. 2014; 436:332–347.
Article29. Ruiz C, Casarejos MJ, Gomez A, Solano R, de Yebenes JG, Mena MA. Protection by glia-conditioned medium in a cell model of Huntington disease. PLoS Curr. 2012; 4:e4fbca54a2028b.
Article30. Saito M, Sakagami H, Fujisawa S. Cytotoxicity and apoptosis induction by butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT). Anticancer Res. 2003; 23:4693–4701.31. Sandoval-Acuña C, Ferreira J, Speisky H. Polyphenols and mitochondria: an update on their increasingly emerging ROS-scavenging independent actions. Arch Biochem Biophys. 2014; 559:75–90.
Article32. Sarada SK, Himadri P, Ruma D, Sharma SK, Pauline T, Mrinalini . Selenium protects the hypoxia induced apoptosis in neuroblastoma cells through upregulation of Bcl-2. Brain Res. 2008; 1209:29–39.
Article33. Tribble DL, Aw TY, Jones DP. The pathophysiological significance of lipid peroxidation in oxidative cell injury. Hepatology. 1987; 7:377–386.
Article34. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39:44–84.
Article35. Vandghanooni S, Forouharmehr A, Eskandani M, Barzegari A, Kafil V, Kashanian S, Ezzati Nazhad Dolatabadi J. Cytotoxicity and DNA fragmentation properties of butylated hydroxyanisole. DNA Cell Biol. 2013; 32:98–103.
Article36. Wang CC, Fang KM, Yang CS, Tzeng SF. Reactive oxygen species-induced cell death of rat primary astrocytes through mitochondria-mediated mechanism. J Cell Biochem. 2009; 107:933–943.
Article37. Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001; 15:2922–2933.38. Weng D, Lu Y, Wei Y, Liu Y, Shen P. The role of ROS in microcystin-LR-induced hepatocyte apoptosis and liver injury in mice. Toxicology. 2007; 232:15–23.
Article39. Xu S, Chen F, Deng M, Sui Y. Simple simultaneous determination of butylated hydroquinone (TBHQ) and butylated hydroxyanisole (BHA) antioxidants in oil using high-performance liquid chromatography with chemiluminescence detection. Luminescence. 2014; 29:1027–1032.
Article40. Xue T, Luo P, Zhu H, Zhao Y, Wu H, Gai R, Wu Y, Yang B, Yang X, He Q. Oxidative stress is involved in Dasatinib-induced apoptosis in rat primary hepatocytes. Toxicol Appl Pharmacol. 2012; 261:280–291.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Induced Heat Shock Protein 33 in Human Corneal Epithelial Cell
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells
- Role of Ascorbic Acid Against the Oxidative Stress in Trabecular Meshwork Cells
- Activation of Caspase-3 in Hydrogen Peroxide-Induced Apoptosis of Human Leukemia HL 60 Cells
- Protective Effects of Peroxiredoxin on Hydrogen Peroxide Induced Oxidative Stress and Apoptosis in Cardiomyocytes