Korean Circ J.
2012 Jan;42(1):23-32. 10.4070/kcj.2012.42.1.23.
Protective Effects of Peroxiredoxin on Hydrogen Peroxide Induced Oxidative Stress and Apoptosis in Cardiomyocytes
- Affiliations
-
- 1Regional Cardiovascular Disease Center, Department of Internal Medicine, Chungbuk National University School of Medicine, Cheongju, Korea. drcorazon@hanmail.net
- KMID: 2297904
- DOI: http://doi.org/10.4070/kcj.2012.42.1.23
Abstract
- BACKGROUND AND OBJECTIVES
The redox system is an important anti-oxidative system composed of thioredoxin, thioredoxin reductase, and peroxiredoxin (PRx). The fine details of PRx expression and its protective effects in various cells in cardiovascular tissue under oxidative stress created by hydrogen peroxide have not been fully elucidated.
SUBJECTS AND METHODS
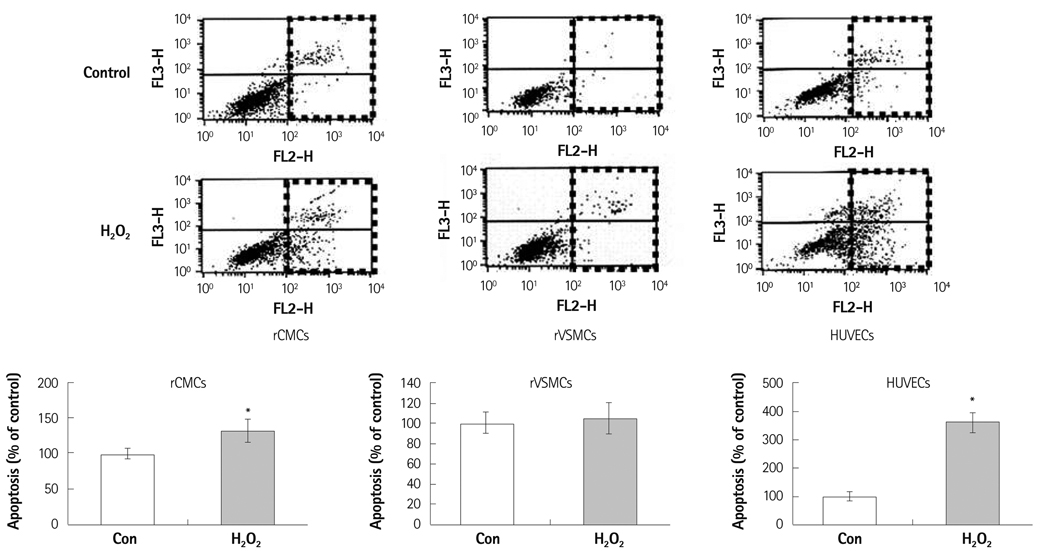
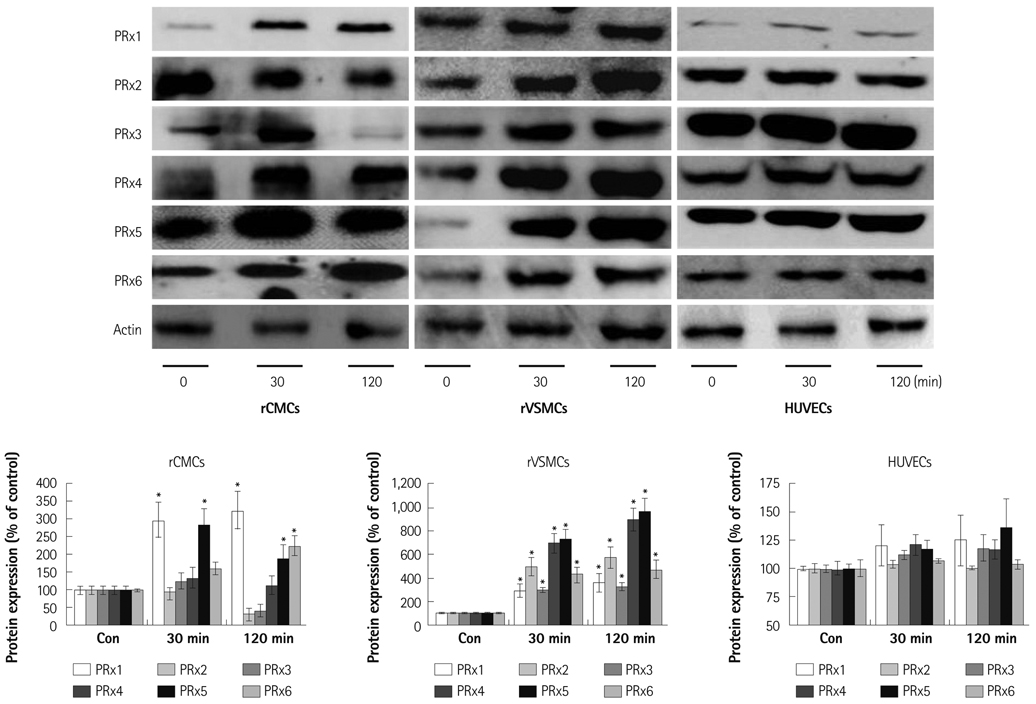
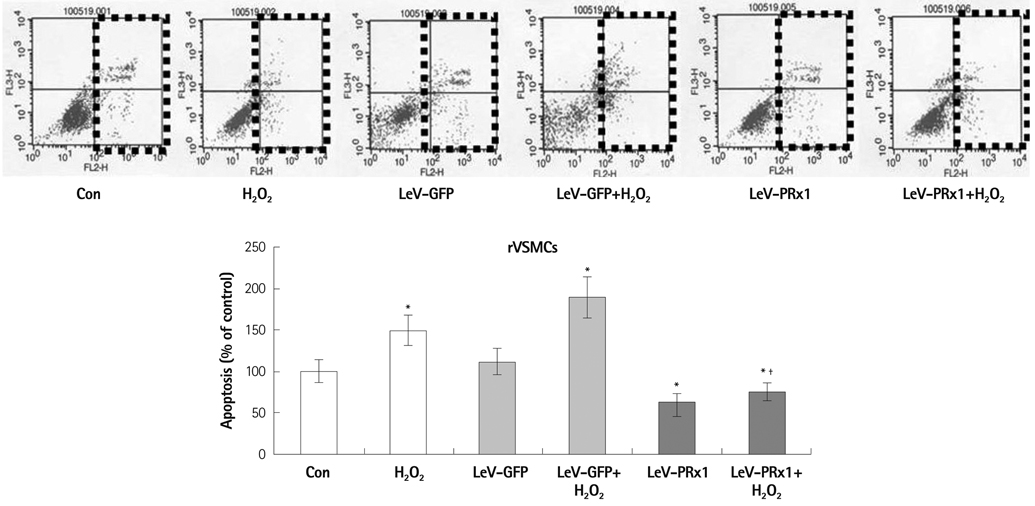
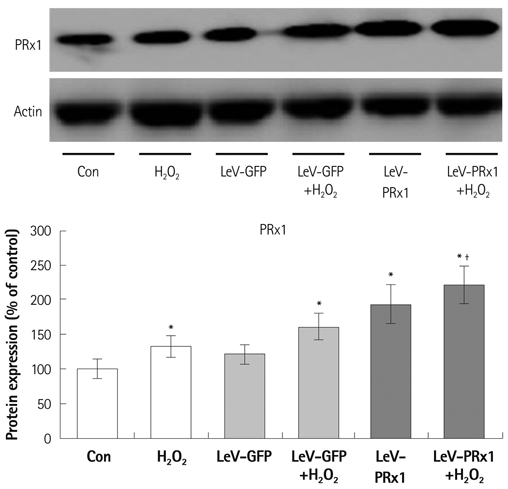
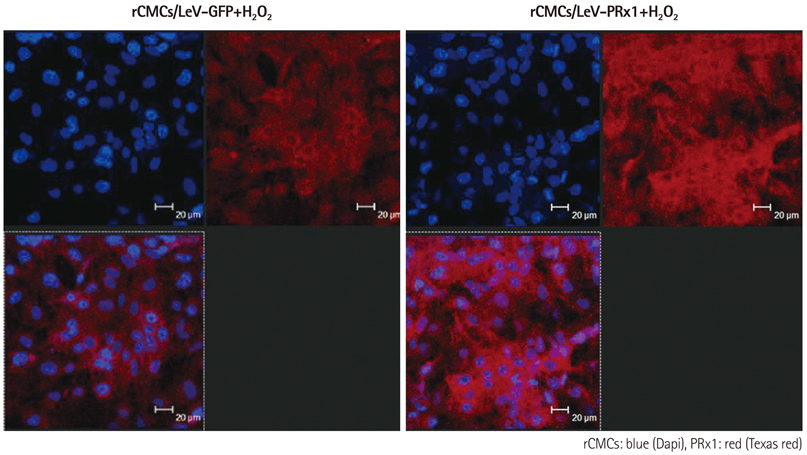
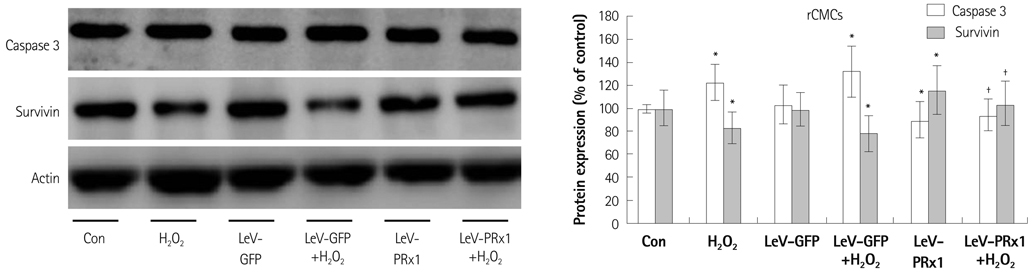
Oxidative stress was induced by adding hydrogen peroxide at 0.25 mM for 2 hours to rat neonatal cardiomyocytes (rCMCs), rat vascular smooth muscle cells (rVSMCs), and human umbilical vein endothelial cells (HUVECs). Apoptosis was quantified by flow cytometry and the expression patterns of the six PRx isoforms were evaluated by western blotting in the three cell lines after hydrogen peroxide stimulation. Apoptosis and the cell survival signal pathway were evaluated by PRx1 gene delivery using lentiviral vector in hydrogen peroxide stimulated rCMCs versus green fluorescence protein gene delivery.
RESULTS
Hydrogen peroxide induced 25% apoptosis in rCMCs. Furthermore, the PRx1 and 5 isoforms were found to be overexpressed in hydrogen peroxide treated rCMCs, and PRx1 overexpression by gene delivery was found to reduce hydrogen peroxide induced rCMCs apoptosis significantly. In addition, this effect was found to originate from cell survival pathway modification.
CONCLUSION
Hydrogen peroxide induced significant oxidative stress in rCMCs, rVSMCs, and HUVECs, and PRx1 overexpression using a lentiviral vector system significantly reduced hydrogen peroxide induced rCMCs apoptosis by upregulation of cell survival signals and downregulation of apoptotic signals. These findings suggest that PRx1 could be used as a treatment strategy for myocardial salvage in conditions of oxidative stress.
Keyword
MeSH Terms
-
Animals
Apoptosis
Blotting, Western
Cell Line
Cell Survival
Down-Regulation
Flow Cytometry
Fluorescence
Human Umbilical Vein Endothelial Cells
Hydrogen
Hydrogen Peroxide
Muscle, Smooth, Vascular
Myocytes, Cardiac
Oxidation-Reduction
Oxidative Stress
Peroxiredoxins
Protein Isoforms
Rats
Signal Transduction
Thioredoxin-Disulfide Reductase
Thioredoxins
Up-Regulation
Hydrogen
Hydrogen Peroxide
Peroxiredoxins
Protein Isoforms
Thioredoxin-Disulfide Reductase
Thioredoxins
Figure
Reference
-
1. Tuteja N, Singh MB, Misra MK, Bhalla PL, Tuteja R. Molecular mechanisms of DNA damage and repair: progress in plants. Crit Rev Biochem Mol Biol. 2001. 36:337–397.2. Tuteja N, Tuteja R. Unravelling DNA repair in human: molecular mechanisms and consequences of repair defect. Crit Rev Biochem Mol Biol. 2001. 36:261–290.3. Tuteja N, Ahmad P, Panda BB, Tuteja R. Genotoxic stress in plants: shedding light on DNA damage, repair and DNA repair helicases. Mutat Res. 2009. 681:134–149.4. Kevin LG, Novalija E, Stowe DF. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth Analg. 2005. 101:1275–1287.5. Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007. 100:460–473.6. Boersma E, Mercado N, Poldermans D, Gardien M, Vos J, Simoons ML. Acute myocardial infarction. Lancet. 2003. 361:847–858.7. Byrne JA, Grieve DJ, Cave AC, Shah AM. Oxidative stress and heart failure. Arch Mal Coeur Vaiss. 2003. 96:214–221.8. Ferrari R, Guardigli G, Mele D, Percoco GF, Ceconi C, Curello S. Oxidative stress during myocardial ischaemia and heart failure. Curr Pharm Des. 2004. 10:1699–1711.9. Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987. 84:1404–1407.10. Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009. 15:RA209–RA219.11. Park KJ, Kim YJ, Choi EJ, et al. Expression pattern of the thioredoxin system in human endothelial progenitor cells and endothelial cells under hypoxic injury. Korean Circ J. 2010. 40:651–658.12. Chae HZ, Robison K, Poole LB, Church G, Storz G, Rhee SG. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci U S A. 1994. 91:7017–7021.13. Hofmann B, Hecht HJ, Flohé L. Peroxiredoxins. Biol Chem. 2002. 383:347–364.14. Poindexter BJ, Smith JR, Buja LM, Bick RJ. Calcium signaling mechanisms in dedifferentiated cardiac myocytes: comparison with neonatal and adult cardiomyocytes. Cell Calcium. 2001. 30:373–382.15. Kobayashi S, Lackey T, Huang Y, et al. Transcription factor gata4 regulates cardiac BCL2 gene expression in vitro and in vivo. FASEB J. 2006. 20:800–802.16. Kau TR, Schroeder F, Ramaswamy S, et al. A chemical genetic screen identifies inhibitors of regulated nuclear export of a forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003. 4:463–476.17. Hoshi H, McKeehan WL. Brain- and liver cell-derived factors are required for growth of human endothelial cells in serum-free culture. Proc Natl Acad Sci U S A. 1984. 81:6413–6417.18. Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation. 1997. 95:320–323.19. Zeng L, Planelles V, Sui Z, et al. HIV-1-based defective lentiviral vectors efficiently transduce human monocytes-derived macrophages and suppress replication of wild-type HIV-1. J Gene Med. 2006. 8:18–28.20. Chen HW, Chien CT, Yu SL, Lee YT, Chen WJ. Cyclosporine A regulate oxidative stress-induced apoptosis in cardiomyocytes: mechanisms via ROS generation, iNOS and Hsp70. Br J Pharmacol. 2002. 137:771–781.21. Penna C, Mancardi D, Tullio F, Pagliaro P. Postconditioning and intermittent bradykinin induced cardioprotection require cyclooxygenase activation and prostacyclin release during reperfusion. Basic Res Cardiol. 2008. 103:368–377.22. Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000. 192:1001–1014.23. Ellis HR, Poole LB. Novel application of 7-chloro-4-nitrobenzo-2-oxa-1, 3-diazole to identify cysteine sulfenic acid in the AhpC component of alkyl hydroperoxide reductase. Biochemistry. 1997. 36:15013–15018.24. Hirotsu S, Abe Y, Okada K, et al. Crystal structure of a multifunctional 2-Cys peroxiredoxin heme-binding protein 23 kDa/proliferation-associated gene product. Proc Natl Acad Sci U S A. 1999. 96:12333–12338.25. Hofmann B, Hecht HJ, Flohé L. Peroxiredoxins. Biol Chem. 2002. 383:347–364.26. Wood ZA, Schröder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003. 28:32–40.27. Prospéri MT, Ferbus D, Karczinski I, Goubin G. A human cDNA corresponding to a gene overexpressed during cell proliferation encodes a product sharing homology with amoebic and bacterial proteins. J Biol Chem. 1993. 268:11050–11056.28. Izumi Y, Kim-Mitsuyama S, Yoshiyama M, et al. Important role of apoptosis signal-regulating kinase 1 in ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2005. 25:1877–1883.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Induced Heat Shock Protein 33 in Human Corneal Epithelial Cell
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells
- Role of Ascorbic Acid Against the Oxidative Stress in Trabecular Meshwork Cells
- Protective Role of Prx(Peroxiredoxin) I and II against H2O2-Induced Apoptosis of MCF7 Cell Lines
- Effect of Ascorbic Acid Against the Oxidative Stress-Induced Cellular Senescence in Trabecular Meshwork Cells