J Korean Med Sci.
2005 Dec;20(6):1039-1045. 10.3346/jkms.2005.20.6.1039.
The Effects of Intradermal Vaccination with DNA Encoding for the T-cell Receptor on the Induction of Experimental Autoimmune Encephalomyelitis in B10.PL Mice
- Affiliations
-
- 1Department of Internal Medicine, The Catholic University of Korea, College of Medicine, Seoul, Korea. sskwon@catholic.ac.kr
- 2Division of Allergy and Immunology, Department of Medicine, University of Tennessee, Memphis, U.S.A.
- KMID: 2157759
- DOI: http://doi.org/10.3346/jkms.2005.20.6.1039
Abstract
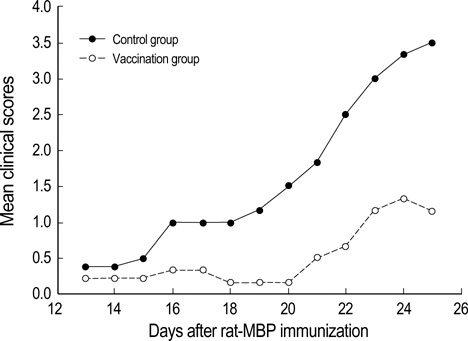
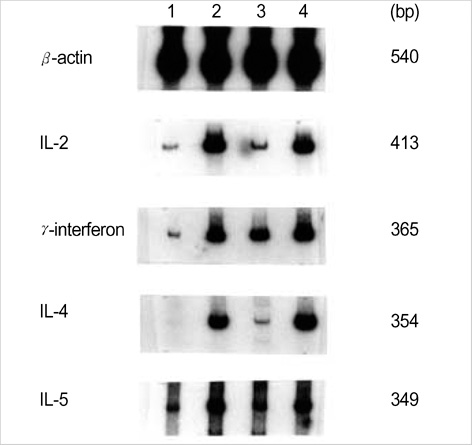
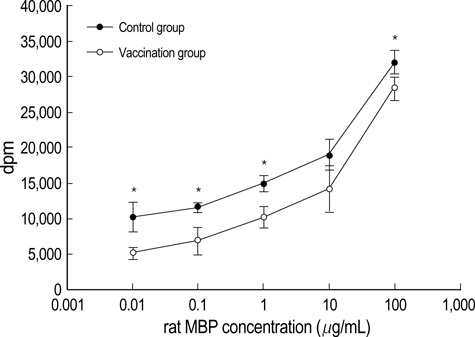
- Intradermal gene administration was found to induce a more profound immune response than direct intramusclular gene injection. We performed intradermal vaccination of B10.PL mice with DNA encoding for the V 8.2 region of the T-cell receptors (TCR). Three weeks later, these mice were immunized with rat myelin basic protein (MBP). Daily mean clinical scores and mortality rate were lower in this group compared with controls. The proliferative responses of lymph node cells to rat MBP were slightly less in the vaccination groups than in the control groups (p<0.05). However, we detected no differences between the two groups with regard to the production of MBP-specific IgG, IgG1, & IgG2a antibodies. The levels of cytokine mRNA expression in the vaccination groups were observed higher than in the control groups without antigen-specific stimulation, but all of cytokine expressions between the vaccination and control groups after antigen-specific stimulation were identical. These results demonstrate that intradermal DNA vaccines encoding for TCR might prove to be useful in the control of autoimmune disease.
Keyword
MeSH Terms
-
Animals
Autoantibodies/blood
Base Sequence
Cytokines/genetics
DNA, Complementary/genetics
Encephalomyelitis, Autoimmune, Experimental/etiology/immunology/*prevention and control
Female
Gene Expression
*Genes, T-Cell Receptor beta
In Vitro
Injections, Intradermal
Lymphocyte Activation
Mice
Myelin Basic Proteins/immunology
RNA, Messenger/genetics/metabolism
Rats
Research Support, N.I.H., Extramural
Research Support, Non-U.S. Gov't
Vaccines, DNA/*administration and dosage/genetics
Figure
Reference
-
1. Mor F, Kantorowitz M, Cohen IR. The dominant and the cryptic T cell repertoire to myelin basic protein in the Lewis rat. J Neurosci Res. 1996. 45:670–679.
Article2. Cheng KC, Chiang HJ, Wang K, Krug MS, Yoo TJ, Hood L. TCRV and TCRJ gene usage in MBP responding T cells from (B10.PL×PL/J) F1 mice is biased towards that of B10.PL mice. J Neuroimmunol. 1997. 80:13–22.3. Matsumoto Y, Tsuchida M, Hanawa J, Abo T. T cell receptor peptide therapy for autoimmune encephalomyelitis: stronger immunization is necessary for effective vaccination. Cell Immunol. 1994. 153:468–478.
Article4. Bot A, Bot S, Antohi S, Karjalainen K, Bona C. Kinetics of generation and persistence on membrane class II molecules of a viral peptide expressed on foreign and self proteins. J Immunol. 1996. 157:3436–3442.5. Raz E, Carson DA, Parker SE, Parr TB, Abai AM, Aichinger G, Gromkowski SH, Singh M, Lew D, Yankauckas MA, Baird SM, Rhodes GH. Intradermal gene immunization: The possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994. 91:9519–9523.
Article6. Kwon SS, Kim N, Yoo TJ. The effect of vaccination with DNA encoding murine T-cell epitopes on the Der p 1 and 2 induced immunoglobulin E synthesis. Allergy. 2001. 56:741–748.
Article7. Waisman A, Ruiz PJ, Hirschberg DL, Gelman A, Oksenberg JR, Brocke S, Mor F, Cohen IR, Steinman L. Suppressive vaccination with DNA encoding a variable region gene of the T-cell receptor prevents autoimmune encephalomyelitis and activates Th2 immunity. Nature Med. 1996. 2:857–859.
Article8. Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991. 9:271–296.
Article9. Smith ME. An in vitro system for the study of myelin synthesis. J Neurochem. 1969. 16:83–92.
Article10. Watanabe T, Cheng KC, Krug MS, Yoo TJ. Brain stem auditory-evoked potentials of mice with experimental allergic encephalomyelitis. Ann Otol Rhinol Laryngol. 1996. 105:905–915.
Article11. Chen KC, Lee KM, Krug MS, Watanabe T, Suzuki M, Choe IS, Yoo TJ. Houst dust mite-induced sensitivity in mice. J Allergy Clin Immunol. 1998. 101:51–59.12. Gorelik L, Flavell RA. Abrogation of TGF-beta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000. 12:171–181.13. Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990. 247:1465–1468.
Article14. Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995. 32:121–182.
Article15. Lobell A, Weissert R, Storch MK, Svanholm C, de Graaf KL, Lassmann H, Andersson R, Olsson T, Wigzell H. Vaccination with DNA encoding an immunodominant myelin basic protein peptide targeted to Fc receptor of immunoglobulin G suppresses experimental autoimmune encephalomyelitis. J Exp Med. 1998. 187:1543–1548.16. Genain CP, Abel K, Belmar N, Villinger F, Rosenberg DP, Linington C, Raine CS, Hauser SL. Late complications of immune deviation therapy in a nonhuman primate. Science. 1996. 274:2054–2057.
Article17. Marusic S, Tonegawa S. Tolerance induction and autoimmune encephalomyelitis amelioration after administration of myelin basic protein-derived peptide. J Exp Med. 1997. 186:507–515.
Article18. Singer GG, Abbas AK. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994. 1:365–371.
Article19. Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998. 19:89–97.
Article20. Kumar V, Sercarz E. Self-determinant selection and selective regulation. Chem Immunol. 1995. 60:1–19.
Article21. Furtado GC, Olivares-Villagomez D, Curotto de Lafaille MA, Wensky AK, Latkowski J, Lafaille JJ. Regulatory T cells in spontaneous autoimmune encephalomyelitis. Immunol Rev. 2001. 182:122–134.
Article22. Mathisen PM, Tuohy VK. Gene therapy in experimental autoimmune encephalomyelitis. J Clin Immunol. 2000. 20:327–333.23. Jiang H., Curran S, Ruiz-Vazquez E, Liang B, Winchester R, Chess L. Regulatory CD8+ T cells fine-tune the myelin basic protein-reactive T cell receptor Vβrepertoire during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2003. 100:8378–8383.24. Braciak TA, Pedersen B, Chin J, Hsiao C, Ward ES, Maricic I, Jahng A, Graham FL, Gauldie J, Sercarz EE, Kumar V. Protection against experimental autoimmune encephalomyelitis generated by a recombinant adenovirus vector expressing the Vβ 8.2 TCR is disrupted by coadministration with vectors expressing either IL-4 or IL-10. J Immunol. 2003. 170:765–774.25. Wefer J, Harrisa RA, Lobell A. Protective DNA vaccination against experimental autoimmune encephalomyelitis is associated with induction of IFN-β. J Neuroimmunol. 2004. 149:66–76.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity and efficacy of a plasmid DNA rabies vaccine incorporating Myd88 as a genetic adjuvant
- DNA-mediated immunization of mice with plasmid encoding HBs antigen
- Experimental autoimmune encephalomyelitis in cynomolgus monkeys
- Natural killer T cell and pathophysiology of asthma
- Erythropoietin and autoimmune neuroinflammation: lessons from experimental autoimmune encephalomyelitis and experimental autoimmune neuritis