Ann Dermatol.
2015 Dec;27(6):709-714. 10.5021/ad.2015.27.6.709.
Impact of Different Spa Waters on Inflammation Parameters in Human Keratinocyte HaCaT Cells
- Affiliations
-
- 1Department of Dermatology, Venereology and Allergology, Johann Wolfgang Goethe University, Frankfurt am Main, Germany. kippenberger@em.uni-frankfurt.de
- 2Kinematic Cell Research Group, Johann Wolfgang Goethe University, Frankfurt am Main, Germany.
- KMID: 2157447
- DOI: http://doi.org/10.5021/ad.2015.27.6.709
Abstract
- BACKGROUND
The treatment of different skin conditions with spa waters is a long tradition dating back to at least late Hellenism. Interestingly, independent scientific examinations studying the effect of spa waters are scarce.
OBJECTIVE
In the present in vitro study, we compared the effect of culture media supplemented with (a) thermal spa waters (La Roche-Posay, Avene) and (b) two natural mineral drinking waters (Heppinger, Adelholzener) on physiological parameters in HaCaT keratinocytes.
METHODS
The different medium preparations were investigated with regard to cell proliferation and cell damage. Moreover, the impact on inflammation parameters with and without ultraviolet B (UVB) irradiation was examined.
RESULTS
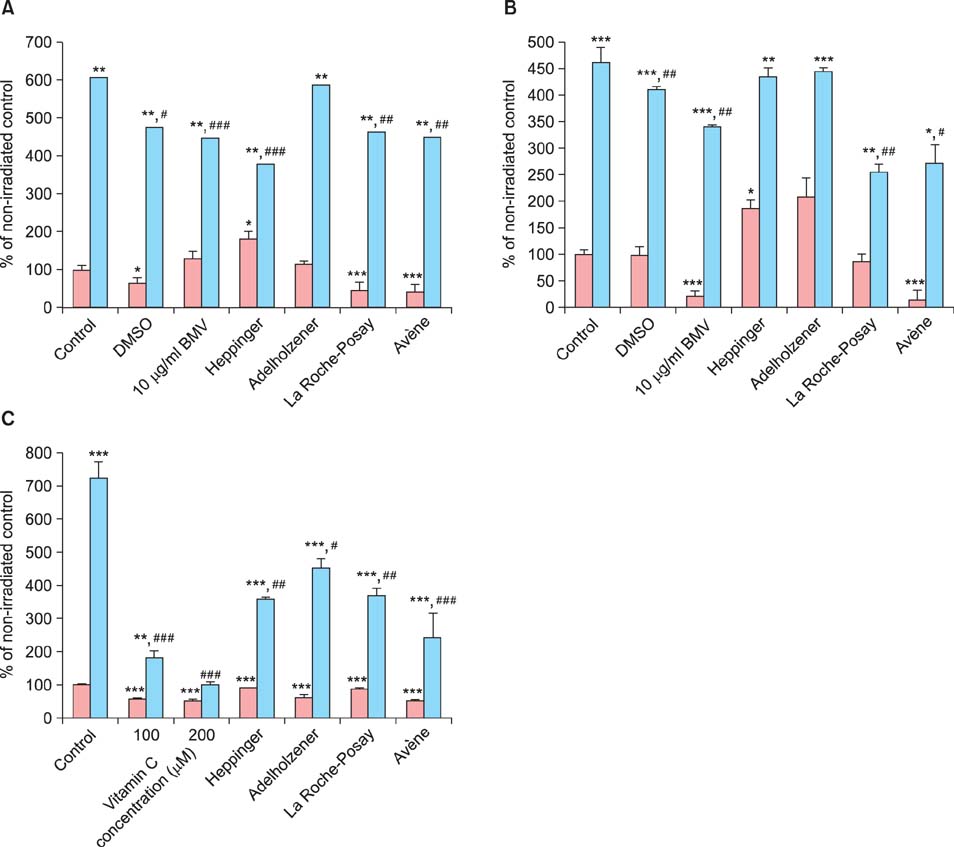
Two popular thermal spring waters were found to suppress cell proliferation and cell damage. Moreover, these waters reversed the induction of interleukin-6, as measured using enzyme-linked immunosorbent assay and promoter transactivation, and the formation of reactive oxygen species after UVB stimulation. Of note, the two natural mineral waters, which are distributed as drinking waters, had some effect on the above-mentioned parameters but to a lesser extent.
CONCLUSION
In summary, our results show that spa waters, and particularly those derived from thermal springs, reduce parameters associated with inflammation. It seems likely that trace elements such as selenium and zinc are critical for the observed effects.
Keyword
MeSH Terms
-
Cell Proliferation
Culture Media
Drinking
Enzyme-Linked Immunosorbent Assay
Humans*
Inflammation*
Interleukin-6
Keratinocytes*
Mineral Waters
Reactive Oxygen Species
Selenium
Skin
Trace Elements
Transcriptional Activation
Water*
Zinc
Culture Media
Interleukin-6
Mineral Waters
Reactive Oxygen Species
Selenium
Trace Elements
Water
Zinc
Figure
Reference
-
1. Sukenik S, Giryes H, Halevy S, Neumann L, Flusser D, Buskila D. Treatment of psoriatic arthritis at the Dead Sea. J Rheumatol. 1994; 21:1305–1309.2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003; 16:132–140.
Article3. Seite S. Thermal waters as cosmeceuticals: La Roche-Posay thermal spring water example. Clin Cosmet Investig Dermatol. 2013; 6:23–28.
Article4. Taieb C, Sibaud V, Merial-Kieny C. Impact of Avène hydrotherapy on the quality of life of atopic and psoriatic patients. J Eur Acad Dermatol Venereol. 2011; 25:Suppl 1. 24–29.
Article5. Tsoureli-Nikita E, Menchini G, Ghersetich I, Hercogova J. Alternative treatment of psoriasis with balneotherapy using Leopoldine spa water. J Eur Acad Dermatol Venereol. 2002; 16:260–262.
Article6. Merial-Kieny C, Mengual X, Guerrero D, Sibaud V. Clinical efficacy of Avène hydrotherapy measured in a large cohort of more than 10,000 atopic or psoriatic patients. J Eur Acad Dermatol Venereol. 2011; 25:Suppl 1. 30–34.
Article7. O'Hare JP, Heywood A, Summerhayes C, Lunn G, Evans JM, Walters G, et al. Observations on the effect of immersion in Bath spa water. Br Med J (Clin Res Ed). 1985; 291:1747–1751.8. Dorn A, Ludwig RJ, Bock A, Thaci D, Hardt K, Bereiter-Hahn J, et al. Oligonucleotides suppress IL-8 in skin keratinocytes in vitro and offer anti-inflammatory properties in vivo. J Invest Dermatol. 2007; 127:846–854.
Article9. Dujic J, Kippenberger S, Hoffmann S, Ramirez-Bosca A, Miquel J, Diaz-Alperi J, et al. Low concentrations of curcumin induce growth arrest and apoptosis in skin keratinocytes only in combination with UVA or visible light. J Invest Dermatol. 2007; 127:1992–2000.
Article10. Plaisance S, Vanden Berghe W, Boone E, Fiers W, Haegeman G. Recombination signal sequence binding protein Jkappa is constitutively bound to the NF-kappaB site of the interleukin-6 promoter and acts as a negative regulatory factor. Mol Cell Biol. 1997; 17:3733–3743.
Article11. Joly F, Charveron M, Ariès MF, Bidault J, Kahhak L, Beauvais F, et al. Effect of Avène spring water on the activation of rat mast cell by substance P or antigen. Skin Pharmacol Appl Skin Physiol. 1998; 11:111–116.
Article12. Staquet MJ, Peguet-Navarro J, Richard A, Schmitt D, Rougier A. In vitro effect of a spa water on the migratory and stimulatory capacities of human Langerhans calls. Eur J Dermatol. 2002; 12:LIX–LXI.13. Lee HP, Choi YJ, Cho KA, Woo SY, Yun ST, Lee JT, et al. Effect of spa spring water on cytokine expression in human keratinocyte HaCaT cells and on differentiation of CD4(+) T cells. Ann Dermatol. 2012; 24:324–336.
Article14. Portalès P, Ariès MF, Licu D, Pinton J, Hernandez-Pion C, Gall Y, et al. Immunomodulation induced by Avène spring water on Th1- and Th2-dependent cytokine production in healthy subjects and atopic dermatitis patients. Skin Pharmacol Appl Skin Physiol. 2001; 14:234–242.
Article15. Cézanne L, Gaboriau F, Charveron M, Morlière P, Tocanne JF, Dubertret L. Effects of the Avène spring water on the dynamics of lipids in the membranes of cultured fibroblasts. Skin Pharmacol. 1993; 6:231–240.
Article16. Lehen'kyi V, Vandenberghe M, Belaubre F, Julié S, Castex-Rizzi N, Skryma R, et al. Acceleration of keratinocyte differentiation by transient receptor potential vanilloid (TRPV6) channel activation. J Eur Acad Dermatol Venereol. 2011; 25:Suppl 1. 12–18.17. Nunes S, Tamura BM. A historical review of mineral water. Surg Cosmet Dermatol. 2012; 4:252–258.18. Prasad AS. Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J Trace Elem Med Biol. 2014; 28:364–371.
Article19. Chebassier N, Ouijja el H, Viegas I, Dreno B. Stimulatory effect of boron and manganese salts on keratinocyte migration. Acta Derm Venereol. 2004; 84:191–194.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vitro Effects of 1,25-Dihydroxyvitamin D3 on the Production of Interleukin-1alpha by Ultraviolet B Irradiation in Cultured Human Keratinocyte Cell Line HaCaT Cells
- Effect of Spa Spring Water on Cytokine Expression in Human Keratinocyte HaCaT Cells and on Differentiation of CD4+ T Cells
- The Effects of Immunosuppressants Rapamycin and Cyclosporin A on the Proliferation, Cell Cycle, and Cyclin Expression of Cultured Human Keratinocyte Cell Line HaCaT Cells
- Organotypic Culture of HaCaT cells: Use of Dermal Substrate that Combines de-epidermized Dermis with Fibroblast-populated Collagen Matrix
- The Production IL-21 and VEGF in UVB-irradiated Human Keratinocyte Cell Line, HaCaT