Effect of Spa Spring Water on Cytokine Expression in Human Keratinocyte HaCaT Cells and on Differentiation of CD4+ T Cells
- Affiliations
-
- 1Department of Dermatology, Uijeongbu St. Mary's Hospital, The Catholic University of Korea College of Medicine, Uijeongbu, Korea. jwkim52@catholic.ac.kr
- 2Department of Microbiology, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Department of Earth and Environmental Sciences, Korea University, Seoul, Korea.
- 4The Korea Central Institute of Hot Spring, Seoul, Korea.
- 5The Korean Academy of Hot Spring, Seoul, Korea.
- 6Department of Dermatology, The Catholic University of Korea College of Medicine, Bucheon, Korea.
- KMID: 2265306
- DOI: http://doi.org/10.5021/ad.2012.24.3.324
Abstract
- BACKGROUND
Skin acts as the first line of defense against any foreign materials outside of our body. In inflammatory skin disease, the pathogenesis is due to an immune reaction in the keratinocytes, immune cells and soluble mediators. Balneotherapy is widely used for the treatment of inflammatory skin disease, but the mechanisms are only partly understood by immune regulation. Balneotherapy in dermatologic disease can affect the secretion of pro-inflammatory cytokines, IL-1alpha and tumor necrosis factor from keratinocytes, and possibly affect the T cell differentiation.
OBJECTIVE
In this study, we evaluated the effect of spa spring water from Yong-gung oncheon on the cells, and investigated the skin immune reaction.
METHODS
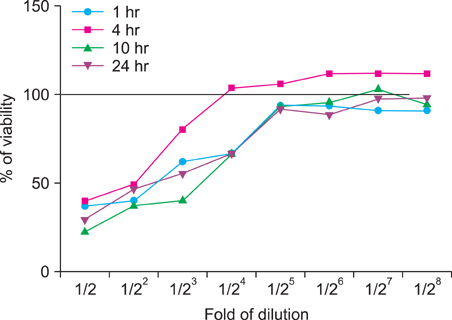
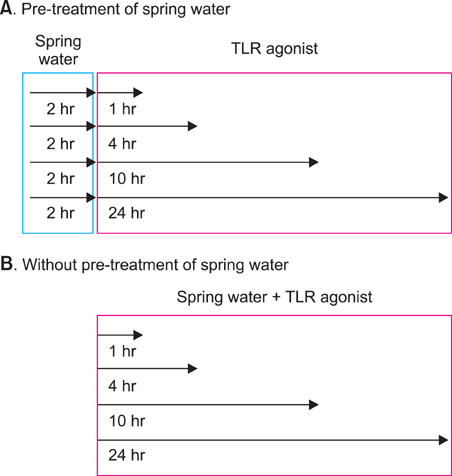
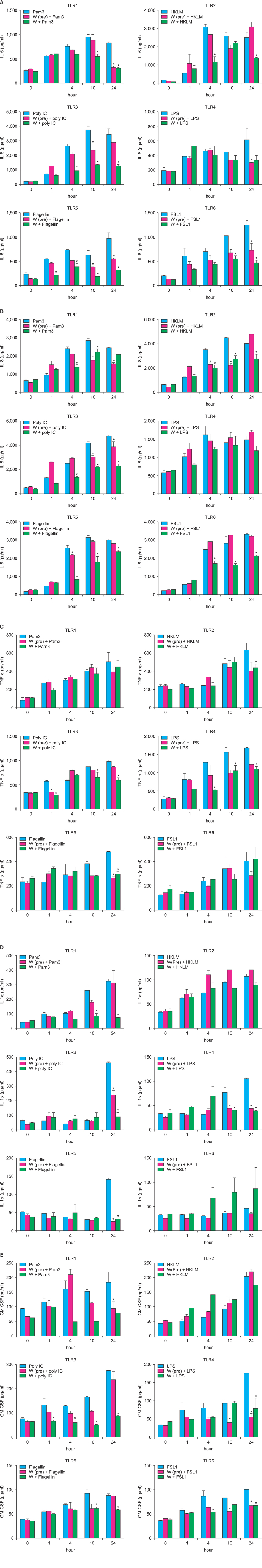
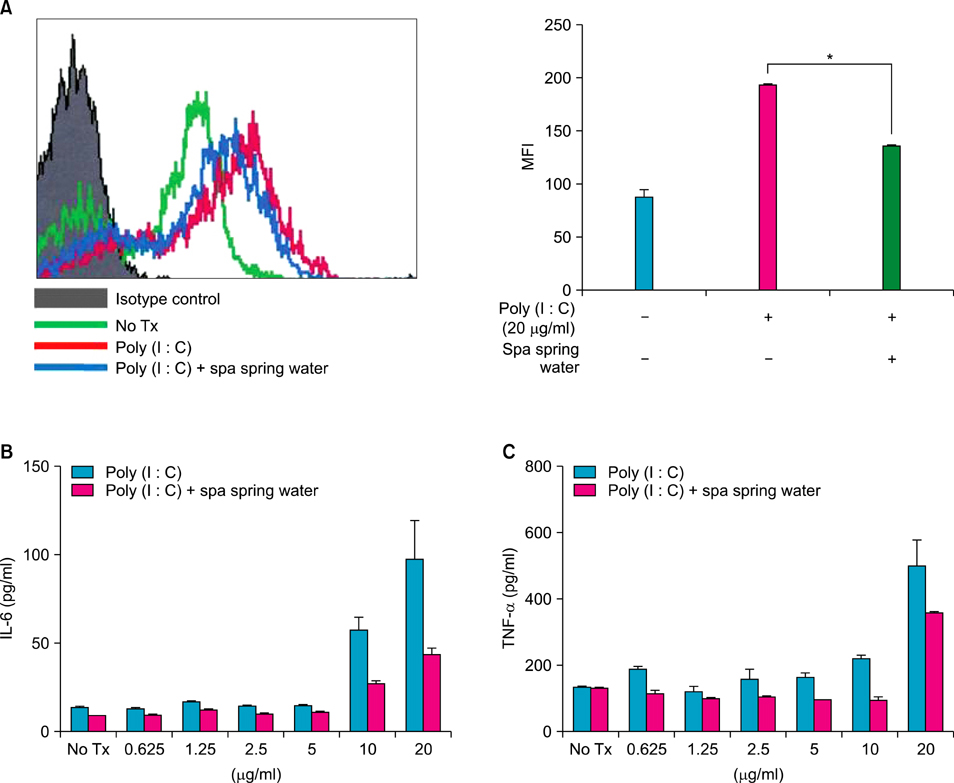
We investigated the immunomodulatory or anti-inflammatory effect of thermal spring water on the expression of pro-inflammatory cytokines in the HaCaT cells under Toll-like receptor (TLR) stimulation, as well as the effect on the differentiation of CD4+ T cells under spring water.
RESULTS
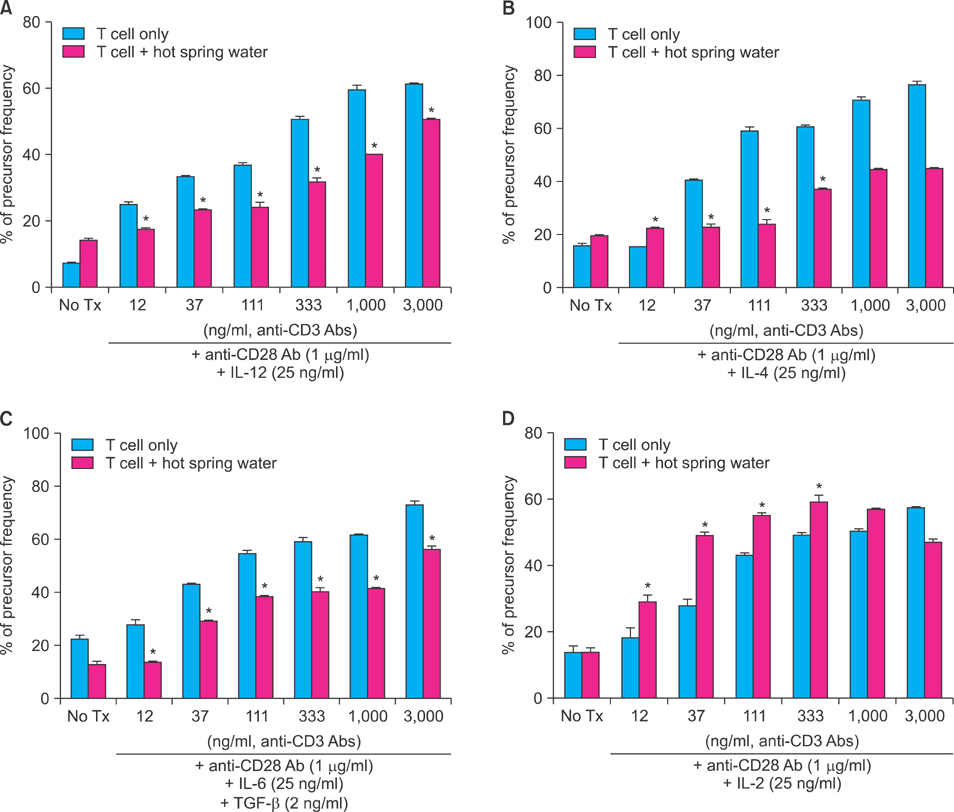
The treatment of spa spring water from Yong-gung oncheon decreased the expression of proinflammatory cytokines under TLR stimulation to the HaCaT cells and antigen presenting cells. In addition, spa spring water attenuated the differentiation process of subsets of CD4+ T cells, i.e., Th1, Th2 and Th17 cells. All these immune parameters can be used to evaluate the efficacy of spa spring water in Korea, in terms of the immune modulatory effect.
CONCLUSION
Spa spring water treatment suppressed the inflammatory cytokines production and also modulated the differentiation of CD4+ T cells into Th1, Th2, and Th17 cells, but not the Tregs cells.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Therapeutic Effects and Immunomodulation of Suanbo Mineral Water Therapy in a Murine Model of Atopic Dermatitis
Yoon Jung Choi, Hye Jin Lee, Do Hyun Lee, So Youn Woo, Kyung Ho Lee, Seong Taek Yun, Jong Moon Kim, Hong Jig Kim, Jin Wou Kim
Ann Dermatol. 2013;25(4):462-470. doi: 10.5021/ad.2013.25.4.462.Immunomodulatory Effects of Balneotherapy with Hae-Un-Dae Thermal Water on Imiquimod-Induced Psoriasis-Like Murine Model
Young Bok Lee, Jun Young Lee, Hye Jin Lee, Seong Taek Yun, Jong Tae Lee, Hong Jig Kim, Dong Soo Yu, So Youn Woo, Jin-Wou Kim
Ann Dermatol. 2014;26(2):221-230. doi: 10.5021/ad.2014.26.2.221.Impact of Different Spa Waters on Inflammation Parameters in Human Keratinocyte HaCaT Cells
Nadja Zöller, Eva Valesky, Matthias Hofmann, Jürgen Bereiter-Hahn, August Bernd, Roland Kaufmann, Markus Meissner, Stefan Kippenberger
Ann Dermatol. 2015;27(6):709-714. doi: 10.5021/ad.2015.27.6.709.Immunomodulatory Effects of Deokgu Thermomineral Water Balneotherapy on Oxazolone-Induced Atopic Dermatitis Murine Model
Young Bok Lee, Su Jin Kim, Sae Mi Park, Kyung Ho Lee, Hyung Jin Han, Dong Soo Yu, So Youn Woo, Seong Taek Yun, Se-Yeong Hamm, Hong Jig Kim, Jin-Wou Kim
Ann Dermatol. 2016;28(2):192-198. doi: 10.5021/ad.2016.28.2.192.
Reference
-
1. Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007. 127:331–341.
Article2. Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009. 129:1339–1350.
Article3. Di Cesare A, Di Meglio P, Nestle FO. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J Invest Dermatol. 2008. 128:2569–2571.
Article4. Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006. 203:2271–2279.
Article5. Albanesi C, Scarponi C, Giustizieri ML, Girolomoni G. Keratinocytes in inflammatory skin diseases. Curr Drug Targets Inflamm Allergy. 2005. 4:329–334.
Article6. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003. 16:132–140.
Article7. Valitutti S, Castellino F, Musiani P. Effect of sulfurous (thermal) water on T lymphocyte proliferative response. Ann Allergy. 1990. 65:463–468.8. Celerier P, Richard A, Litoux P, Dreno B. Modulatory effects of selenium and strontium salts on keratinocyte-derived inflammatory cytokines. Arch Dermatol Res. 1995. 287:680–682.
Article9. Lee KH, Cho KA, Kim JY, Kim JY, Baek JH, Woo SY, et al. Filaggrin knockdown and Toll-like receptor 3 (TLR3) stimulation enhanced the production of thymic stromal lymphopoietin (TSLP) from epidermal layers. Exp Dermatol. 2011. 20:149–151.
Article10. Wiedow O, Streit V, Christophers E, Ständer M. Liberation of human leukocyte elastase by hypertonic saline baths in psoriasis. Hautarzt. 1989. 40:518–522.11. Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008. 159:1092–1102.
Article12. Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005. 174:3695–3702.
Article13. Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010. 10:479–489.
Article14. Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009. 30:636–645.
Article15. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008. 8:523–532.
Article16. Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008. 9:239–244.
Article17. Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010. 11:7–13.
Article18. Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009. 15:1377–1382.
Article19. Kim CK, Choi J, Callaway Z, Iijima K, Volcheck G, Kita H. Increases in airway eosinophilia and a th1 cytokine during the chronic asymptomatic phase of asthma. Respir Med. 2010. 104:1436–1443.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of Different Spa Waters on Inflammation Parameters in Human Keratinocyte HaCaT Cells
- Organotypic Culture of HaCaT cells: Use of Dermal Substrate that Combines de-epidermized Dermis with Fibroblast-populated Collagen Matrix
- In Vitro Effects of 1,25-Dihydroxyvitamin D3 on the Production of Interleukin-1alpha by Ultraviolet B Irradiation in Cultured Human Keratinocyte Cell Line HaCaT Cells
- Interleukin-17 and Interleukin-22 Induced Proinflammatory Cytokine Production in Keratinocytes via Inhibitor of Nuclear Factor kappaB Kinase-alpha Expression
- Expressional Changes of c-fos, c-jun, and c-myc Induced by 12-O-tetradecanoylphorbol 13-acetate (TPA) in HaCaT Cells