Immune Netw.
2010 Apr;10(2):75-80. 10.4110/in.2010.10.2.75.
The Production IL-21 and VEGF in UVB-irradiated Human Keratinocyte Cell Line, HaCaT
- Affiliations
-
- 1Department of Anatomy and Tumor Immunity Medical Research Center, Seoul National University College of Medicine, Seoul 110-799, Korea. kinglee@snu.ac.kr
- KMID: 2150662
- DOI: http://doi.org/10.4110/in.2010.10.2.75
Abstract
-
BACKGROUND: Ultraviolet B (UVB) induces multiple inflammatory and carcinogenic reactions. In skin, UVB induces to secrete several kinds of inflammatory cytokines from keratinocytes and also increases angiogenic process via the modulation of vascular endothelial growth factor (VEGF) production. Interleukin-21 (IL-21) is an inflammatory cytokine and produced by activated T cells. The biologic functions of IL-21 have not yet extensively studied.
METHODS
In the present study, we investigate the production of IL-21 from human keratinocyte cell line, HaCaT and its biological effect after exposure to UVB.
RESULTS
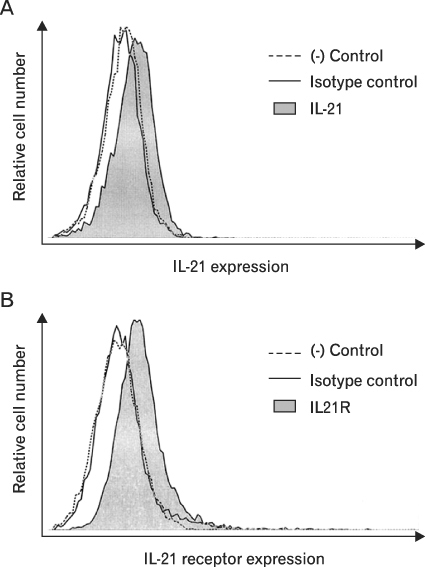
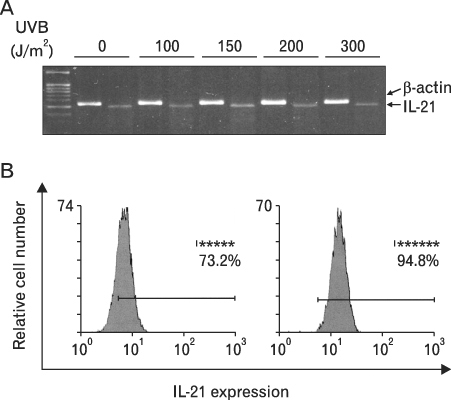
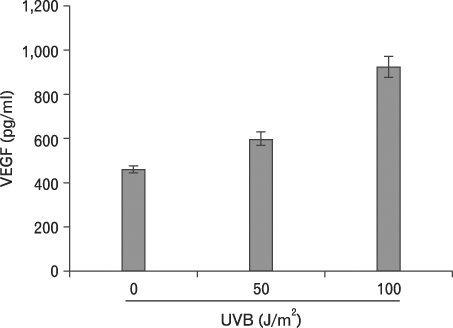
First, we confirmed the IL-21 production and its receptor expression in HaCaT. And then, the change of IL-21 and VEGF production in HaCaT by UVB irradiation was examined. Not only IL-21 but also VEGF production was enhanced by UVB irradiation. Next, to determine relationship of enhanced production of IL-21 and VEGF, we detected VEGF production after neutralization of IL-21. VEGF production was reduced by IL-21 neutralization, which indicates that the IL-21 is involved in the VEGF production.
CONCLUSION
Taken together, our results suggest that IL-21 and VEGF production is enhanced by UVB irradiation in HaCaT. In addition, it seems that IL-21 plays a role in the angiogenic process in skin via the modulation of VEGF production.
MeSH Terms
Figure
Reference
-
1. Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2006. 5:688–698.
Article2. Mehta DS, Wurster AL, Grusby MJ. Biology of IL-21 and the IL-21 receptor. Immunol Rev. 2004. 202:84–95.
Article3. Vosshenrich CA, Di Santo JP. Cytokines: IL-21 joins the gamma (c)-dependent network? Curr Biol. 2001. 11:R175–R177.4. Brandt K, Singh PB, Bulfone-Paus S, Ruckert R. Interleukin-21: a new modulator of immunity, infection, and cancer. Cytokine Growth Factor Rev. 2007. 18:223–232.
Article5. Habib T, senadheera S, Winberg K, Kaushansky K. The common gamma chain (gamma c) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry. 2002. 41:8725–8731.
Article6. Suto A, Wurster AL, Riner SL, Grusby MJ. IL-21 inhibits IFN-gamma production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol. 2006. 177:3721–3727.
Article7. Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007. 109:4135–4142.
Article8. Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci U S A. 2000. 97:11439–11444.
Article9. Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000. 408:57–63.
Article10. Leonard WJ, Zeng R, Spolski R. Interleukin 21: a cytokine/cytokine receptor system that has come of age. J Leukoc Biol. 2008. 84:348–356.
Article11. Sawalha AH, Kaufman KM, Kelly JA, Adler AJ, Aberle T, Kilpatrick J, Wakeland EK, Li QZ, Wandstrat AE, Karp DR, James JA, Merrill JT, Lipsky P, Harley JB. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis. 2008. 67:458–461.
Article12. Young DA, Hegen M, Ma HL, Whitters MJ, Albert LM, Lowe L, Senices M, Wu PW, Sibley B, Leathurby Y, Brown TP, Nickerson-Nutter C, Keith JC Jr, Collins M. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum. 2007. 56:1152–1163.
Article13. Skak K, Kragh M, Hausman D, Smyth MJ, Sivakumar PV. Interleukin 21: combination strategies for cancer therapy. Nat Rev Drug Discov. 2008. 7:231–240.
Article14. Terman BI, Stoletov KV. VEGF and tumor angiogenesis. Einstein Quart J Biol. 2001. 18:59–66.15. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995. 1:27–31.
Article16. Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992. 176:1375–1379.
Article17. Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, Berse B, Dvorak HF. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994. 180:1141–1146.
Article18. Brown LF, Harrist TJ, Yeo KT, Ståhle-Bäckdahl M, Jackman RW, Berse B, Tognazzi K, Dvorak HF, Detmar M. Increased expression of vascular permeability factor (vascular endothelial growth factor) in bullous pemphigoid, dermatitis herpetiformis, and erythema multiforme. J Invest Dermatol. 1995. 104:744–749.
Article19. Sakkoula E, Pipili-Synetos E, Maragoudakis ME. Involvement of nitric oxide in the inhibition of angiogenesis by interleukin-2. Br J Pharmacol. 1997. 122:793–795.
Article20. Volpert OV, Fong T, Koch AE, Peterson JD, Waltenbaugh C, Tepper RI, Bouck NP. Inhibition of angiogenesis by interleukin 4. J Exp Med. 1998. 188:1039–1046.
Article21. Castermans K, Tabruyn SP, Zeng R, van Beijnum JR, Eppolito C, Leonard WJ, Shrikant PA, Griffioen AW. Angiostatic activity of the antitumor cytokine interleukin-21. Blood. 2008. 112:4940–4947.
Article22. Griswold DE, Tzimas MN. Ultraviolet B-induced inflammatory cytokine production, in vivo: initial pharmacological characterization. Inflamm Res. 1995. 44:Suppl 2. S209–S210.23. Kondo S, Kono T, Sauder DN, McKenzie RC. IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J Invest Dermatol. 1993. 101:690–694.
Article24. Bielenberg DR, Bucana CD, Sanchez R, Donawho CK, Kripke ML, Fidler IJ. Molecular regulation of UVB-induced cutaneous angiogenesis. J Invest Dermatol. 1998. 111:864–872.
Article25. Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol. 2005. 152:115–121.
Article26. Nickoloff BJ, Qin JZ, Nestle FO. Immunopathogenesis of psoriasis. Clin Rev Allergy Immunol. 2007. 33:45–56.
Article27. Monteleone G, Pallone F, Macdonald TT. Interleukin-21 (IL-21)-mediated pathways in T cell-mediated disease. Cytokine Growth Factor Rev. 2009. 2:185–191.
Article28. Yoshizumi M, Nakamura T, Kato M, Ishioka T, Kozawa K, Wakamatsu K, Kimura H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int. 2008. 32:1405–1411.
Article29. Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007. 448:480–483.
Article30. Hirakawa S, Fujii S, Kajiya K, Yano K, Detmar M. Vascular endothelial growth factor promotes sensitivity to ultraviolet B-induced cutaneous photodamage. Blood. 2004. 105:2392–2399.
Article31. Brauchle M, Funk JO, Kind P, Werner S. Ultraviolet B and H2O2 are potent inducers of vascular endothelial growth factor expression in cultured keratinocytes. J Biol Chem. 1996. 271:21793–21797.
Article32. Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF Jr, Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006. 116:2044–2055.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vitro Effects of 1,25-Dihydroxyvitamin D3 on the Production of Interleukin-1alpha by Ultraviolet B Irradiation in Cultured Human Keratinocyte Cell Line HaCaT Cells
- Comparison of the Expression Levels of Anti-proliferative Genes between HaCaT Keratinocytes and Squamous Cancer Cell Lines after UVB Irradiation
- Anti-inflammatory Effect of Curcumin on UVB-induced Inflammatory Cytokines in HaCaT Cells
- Expression of Apoptosis Related Genes from HaCaT Cell after UVB Irradiation
- Early Phase of UVB-induced GM-CSF Upregulation in Epithelial Cell Line is not Totally Dependent on IL-1α