J Korean Med Sci.
2015 Jan;30(1):34-43. 10.3346/jkms.2015.30.1.34.
Cardioprotective Effect of Fimasartan, a New Angiotensin Receptor Blocker, in a Porcine Model of Acute Myocardial Infarction
- Affiliations
-
- 1The Heart Research Center of Chonnam National University Hospital Designated by Korea Ministry of Health, Welfare and Family Affairs, Gwangju, Korea. myungho@chollian.net
- 2Department of Nuclear Medicine, Chonnam National University Hospital, Gwangju, Korea.
- 3Department of Cardiothoracic Surgery, Chonnam National University Hospital, Gwangju, Korea.
- KMID: 2155445
- DOI: http://doi.org/10.3346/jkms.2015.30.1.34
Abstract
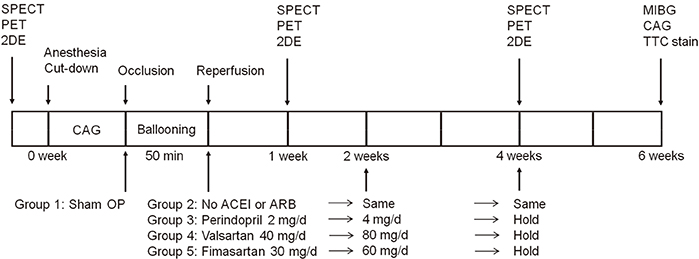
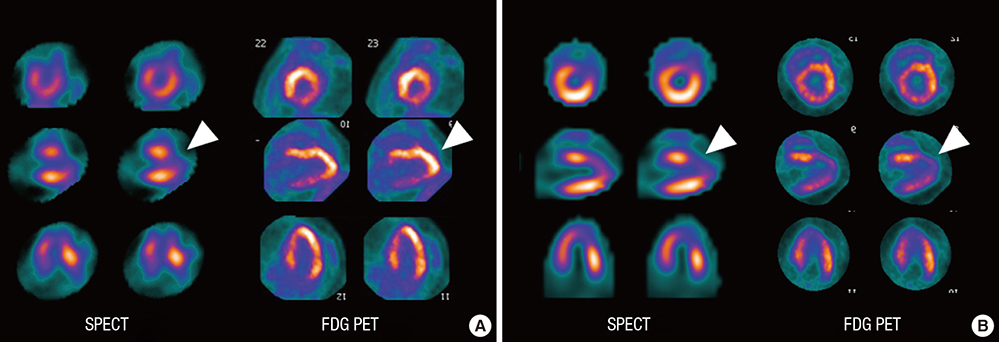
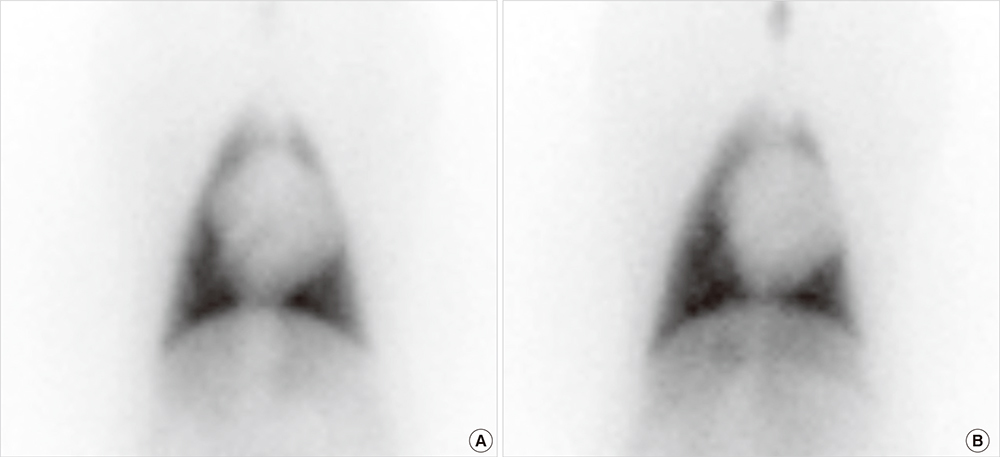
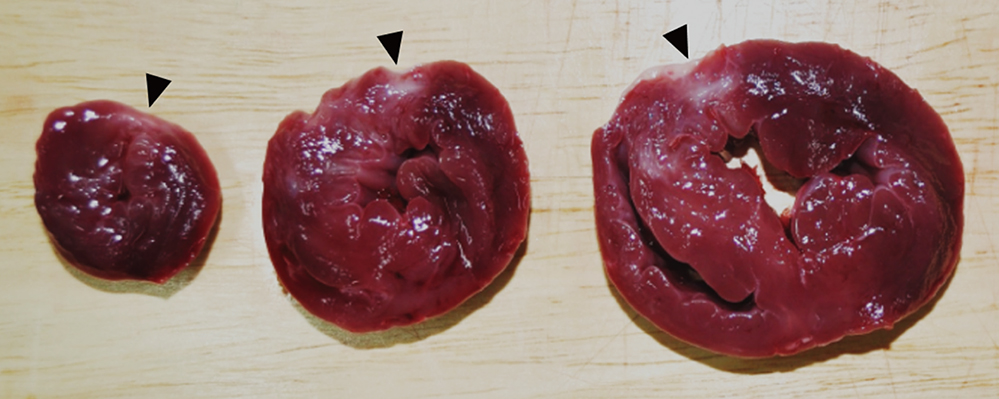
- Cardioprotective effect of fimasartan, a new angiotensin receptor blocker (ARB), was evaluated in a porcine model of acute myocardial infarction (MI). Fifty swine were randomized to group 1 (sham, n=10), group 2 (no angiotensin-converting enzyme inhibitor [ACEI] or ARB, n=10), group 3 (perindopril 2 mg daily, n=10), group 4 (valsartan 40 mg daily, n=10), or group 5 (fimasartan 30 mg daily, n=10). Acute MI was induced by occlusion of the left anterior descending artery for 50 min. Echocardiography, single photon emission computed tomography (SPECT), and F-18 fluorodeoxyglucose cardiac positron emission tomography (PET) were performed at baseline, 1 week, and 4 weeks. Iodine-123 meta-iodobenzylguanidine (MIBG) scan was done at 6 weeks for visualization of cardiac sympathetic activity. Left ventricular function and volumes at 4 weeks were similar between the 5 groups. No difference was observed in groups 2 to 5 in SPECT perfusion defect, matched and mismatched segments between SPECT and PET at 1 week and 4 weeks. MIBG scan showed similar uptake between the 5 groups. Pathologic analysis showed similar infarct size in groups 2 to 5. Infarct size reduction was not observed with use of fimasartan as well as other ACEI and ARB in a porcine model of acute MI.
Keyword
MeSH Terms
-
3-Iodobenzylguanidine
Angiotensin II Type 1 Receptor Blockers/therapeutic use
Angiotensin Receptor Antagonists/*therapeutic use
Angiotensin-Converting Enzyme Inhibitors/therapeutic use
Animals
Anterior Wall Myocardial Infarction/*drug therapy/physiopathology
Biphenyl Compounds/*therapeutic use
Cardiotonic Agents/*therapeutic use
Disease Models, Animal
Echocardiography
Fluorodeoxyglucose F18
Perindopril/therapeutic use
Positron-Emission Tomography
Pyrimidines/*therapeutic use
Random Allocation
Swine
Tetrazoles/*therapeutic use
Tomography, Emission-Computed, Single-Photon
Valsartan/therapeutic use
Ventricular Function, Left/*physiology
3-Iodobenzylguanidine
Angiotensin II Type 1 Receptor Blockers
Angiotensin Receptor Antagonists
Angiotensin-Converting Enzyme Inhibitors
Biphenyl Compounds
Cardiotonic Agents
Perindopril
Pyrimidines
Tetrazoles
Fluorodeoxyglucose F18
Valsartan
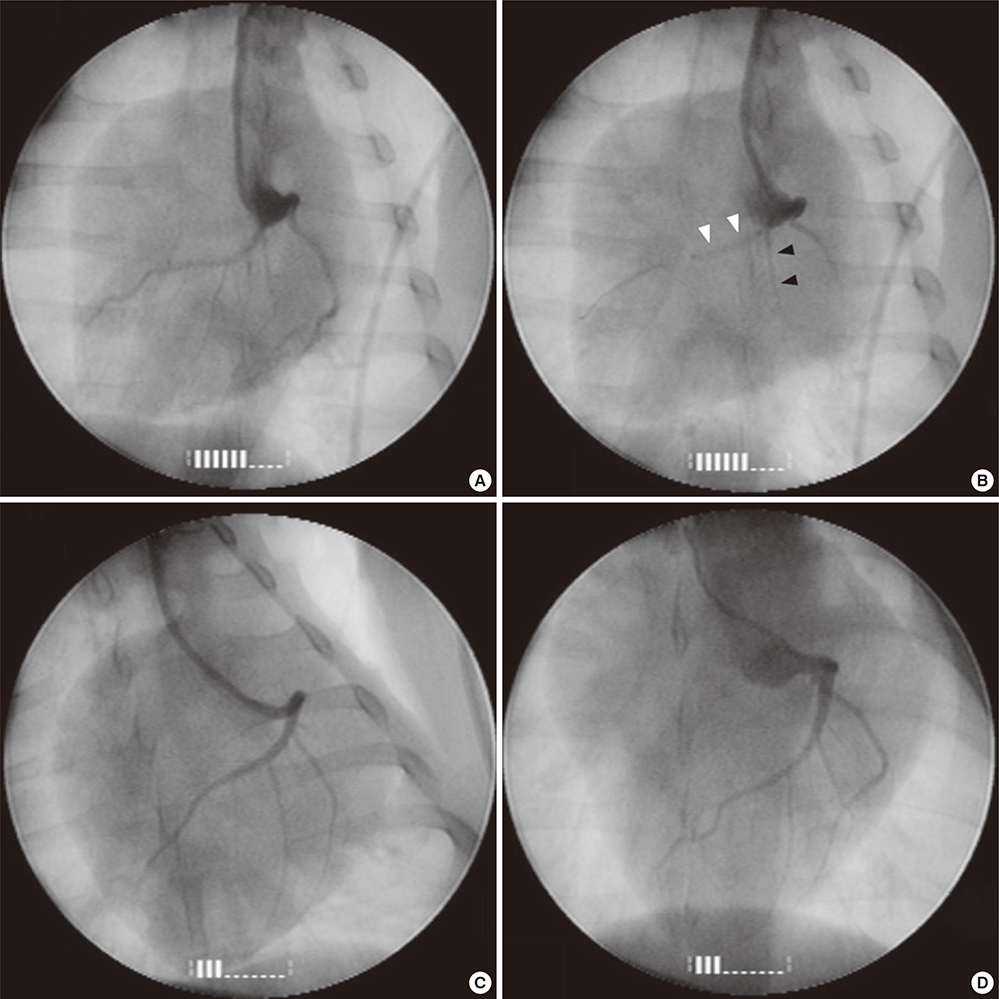
Figure
Cited by 1 articles
-
Therapeutic Effect of Fimasartan in a Rat Model of Myocardial Infarction Evaluated by Cardiac Positron Emission Tomography with [18F]FPTP
Hyukjin Park, Hyeon Sik Kim, Young Joon Hong, Jung-Joon Min, Han Byul Kim, Min Chul Kim, Doo Sun Sim, Ju Han Kim, Dong-Yeon Kim, Jae Sung Lee, Youngkeun Ahn, Myung Ho Jeong
Chonnam Med J. 2019;55(2):109-115. doi: 10.4068/cmj.2019.55.2.109.
Reference
-
1. Jalowy A, Schulz R, Dörge H, Behrends M, Heusch G. Infarct size reduction by AT1-receptor blockade through a signal cascade of AT2-receptor activation, bradykinin and prostaglandins in pigs. J Am Coll Cardiol. 1998; 32:1787–1796.2. Jugdutt BI, Menon V. AT2 receptor and apoptosis during AT1 receptor blockade in reperfused myocardial infarction in the rat. Mol Cell Biochem. 2004; 262:203–214.3. Jugdutt BI, Balghith M. Enhanced regional AT(2)-receptor and PKC (epsilon) expression during cardioprotection induced by AT(1)-receptor blockade after reperfused myocardial infarction. J Renin Angiotensin Aldosterone Syst. 2001; 2:134–140.4. Sato M, Engelman RM, Otani H, Maulik N, Rousou JA, Flack JE 3rd, Deaton DW, Das DK. Myocardial protection by preconditioning of heart with losartan, an angiotensin II type 1-receptor blocker: implication of bradykinin-dependent and bradykinin-independent mechanisms. Circulation. 2000; 102:Iii346–Iii351.5. Lee SE, Kim YJ, Lee HY, Yang HM, Park CG, Kim JJ, Kim SK, Rhee MY, Oh BH. Investigators. Efficacy and tolerability of fimasartan, a new angiotensin receptor blocker, compared with losartan (50/100 mg): a 12-week, phase III, multicenter, prospective, randomized, double-blind, parallel-group, dose escalation clinical trial with an optional 12-week extension phase in adult Korean patients with mild-to-moderate hypertension. Clin Ther. 2012; 34:552–568. 68.e1–68.e9.6. Lee H, Yang HM, Lee HY, Kim JJ, Choi DJ, Seung KB, Jeon ES, Ha JW, Rim SJ, Park JB, et al. Efficacy and tolerability of once-daily oral fimasartan 20 to 240 mg/d in Korean patients with hypertension: findings from Two Phase II, randomized, double-blind, placebo-controlled studies. Clin Ther. 2012; 34:1273–1289.7. Park JB, Sung KC, Kang SM, Cho EJ. Safety and efficacy of fimasartan in patients with arterial hypertension (Safe-KanArb study): an open-label observational study. Am J Cardiovasc Drugs. 2013; 13:47–56.8. Lee H, Kim KS, Chae SC, Jeong MH, Kim DS, Oh BH. Ambulatory blood pressure response to once-daily fimasartan: an 8-week, multicenter, randomized, double-blind, active-comparator, parallel-group study in Korean patients with mild to moderate essential hypertension. Clin Ther. 2013; 35:1337–1349.9. Lee JY, Lee CW, Kim WJ, Ahn JM, Park DW, Kang SJ, Lee SW, Kim YH, Son WC, Jung S, et al. Antiatherosclerotic effects of the novel angiotensin receptor antagonist Fimasartan on plaque progression and stability in a rabbit model: a double-blind placebo-controlled trial. J Cardiovasc Pharmacol. 2013; 62:229–236.10. Han J, Park SJ, Thu VT, Lee SR, Long le T, Kim HK, Kim N, Park SW, Jeon ES, Kim EJ, et al. Effects of the novel angiotensin II receptor type I antagonist, fimasartan on myocardial ischemia/reperfusion injury. Int J Cardiol. 2013; 168:2851–2859.11. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989; 2:358–367.12. Hawk CT, Leary SL, Morris TH. American College of Laboratory Animal Medicine. European College of Laboratory Animal Medicine. Formulary for laboratory animals. 3rd ed. Ames, Iowa: Blackwell Pub.;2005.13. Wang J, Khoury DS, Thohan V, Torre-Amione G, Nagueh SF. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation. 2007; 115:1376–1383.14. American Society of Nuclear Cardiology. Imaging Guidelines Overview: ASNC Imaging Guidelines for Nuclear Cardiology Procedures. accessed on 13 November 2007. Available at http://www.asnc.org/content_1884.cfm.15. Lautamäki R, Schuleri KH, Sasano T, Javadi MS, Youssef A, Merrill J, Nekolla SG, Abraham MR, Lardo AC, Bengel FM. Integration of infarct size, tissue perfusion, and metabolism by hybrid cardiac positron emission tomography/computed tomography: evaluation in a porcine model of myocardial infarction. Circ Cardiovasc Imaging. 2009; 2:299–305.16. Liu YH, Sinusas AJ, Khaimov D, Gebuza BI, Wackers FJ. New hybrid count- and geometry-based method for quantification of left ventricular volumes and ejection fraction from ECG-gated SPECT: methodology and validation. J Nucl Cardiol. 2005; 12:55–65.17. Nuyts J, Mortelmans L, Suetens P, Oosterlinck A, de Rou M. Model-based quantification of myocardial perfusion images from SPECT. J Nucl Med. 1989; 30:1992–2001.18. Jacobson AF, Lombard J, Banerjee G, Camici PG. 123I-mIBG scintigraphy to predict risk for adverse cardiac outcomes in heart failure patients: design of two prospective multicenter international trials. J Nucl Cardiol. 2009; 16:113–121.19. Fonge H, Vunckx K, Wang H, Feng Y, Mortelmans L, Nuyts J, Bormans G, Verbruggen A, Ni Y. Non-invasive detection and quantification of acute myocardial infarction in rabbits using mono-[123I]iodohypericin microSPECT. Eur Heart J. 2008; 29:260–269.20. Dawson B, Trapp RG. Basic & clinical biostatistics. 4th ed. New York: Lange Medical Books-McGraw-Hill, Medical Pub. Division;2004.21. Sim DS, Jeong MH, Kim YH, Choi S, Lim KS, Kim JH, Cho KH, Kim MC, Kim HK, Kim SS, et al. Effects of sildenafil in combination with angiotensin-converting enzyme inhibitor on limiting infarct expansion in a porcine model of acute myocardial infarction. Int J Cardiol. 2011; 146:459–460.22. Weidenbach R, Schulz R, Gres P, Behrends M, Post H, Heusch G. Enhanced reduction of myocardial infarct size by combined ACE inhibition and AT(1)-receptor antagonism. Br J Pharmacol. 2000; 131:138–144.23. Messadi-Laribi E, Griol-Charhbili V, Gaies E, Vincent MP, Heudes D, Meneton P, Alhenc-Gelas F, Richer C. Cardioprotection and kallikrein-kinin system in acute myocardial ischaemia in mice. Clin Exp Pharmacol Physiol. 2008; 35:489–493.24. Messadi-Laribi E, Griol-Charhbili V, Pizard A, Vincent MP, Heudes D, Meneton P, Alhenc-Gelas F, Richer C. Tissue kallikrein is involved in the cardioprotective effect of AT1-receptor blockade in acute myocardial ischemia. J Pharmacol Exp Ther. 2007; 323:210–216.25. Safari F, Hajizadeh S, Shekarforoush S, Bayat G, Foadoddini M, Khoshbaten A. Influence of ramiprilat and losartan on ischemia reperfusion injury in rat hearts. J Renin Angiotensin Aldosterone Syst. 2012; 13:29–35.26. Black SC, Driscoll EM, Lucchesi BR. Effect of ramiprilat or captopril on myocardial infarct size: assessment in canine models of ischemia alone and ischemia with reperfusion. Pharmacology. 1998; 57:35–46.27. Lange SA, Wolf B, Schober K, Wunderlich C, Marquetant R, Weinbrenner C, Strasser RH. Chronic angiotensin II receptor blockade induces cardioprotection during ischemia by increased PKC-epsilon expression in the mouse heart. J Cardiovasc Pharmacol. 2007; 49:46–55.28. Xu J, Carretero OA, Shesely EG, Rhaleb NE, Yang JJ, Bader M, Yang XP. The kinin B1 receptor contributes to the cardioprotective effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in mice. Exp Physiol. 2009; 94:322–329.29. Zhu B, Sun Y, Sievers RE, Browne AE, Lee RJ, Chatterjee K, Parmley WW. Effects of different durations of pretreatment with losartan on myocardial infarct size, endothelial function, and vascular endothelial growth factor. J Renin Angiotensin Aldosterone Syst. 2001; 2:129–133.30. Zhu B, Sun Y, Sievers RE, Browne AE, Pulukurthy S, Sudhir K, Lee RJ, Chou TM, Chatterjee K, Parmley WW. Comparative effects of pretreatment with captopril and losartan on cardiovascular protection in a rat model of ischemia-reperfusion. J Am Coll Cardiol. 2000; 35:787–795.31. ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008; 358:1547–1559.32. Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, et al. ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008; 372:547–553.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Effect of Fimasartan in a Rat Model of Myocardial Infarction Evaluated by Cardiac Positron Emission Tomography with [¹â¸F]FPTP
- Comparison Between Fimasartan Versus Other Angiotensin Receptor Blockers in Patients With Heart Failure After Acute Myocardial Infarction
- Fimasartan, an angiotensin II receptor antagonist, ameliorates an in vivo zebrafish model of heart failure
- Decreased potency of fimasartan in liver cirrhosis was quantified using mixed-effects analysis
- Angiotensin II Type 1 Receptor Blocker, Fimasartan, Reduces Vascular Smooth Muscle Cell Senescence by Inhibiting the CYR61 Signaling Pathway