Korean Circ J.
2019 Jul;49(7):615-626. 10.4070/kcj.2018.0379.
Angiotensin II Type 1 Receptor Blocker, Fimasartan, Reduces Vascular Smooth Muscle Cell Senescence by Inhibiting the CYR61 Signaling Pathway
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. hylee612@snu.ac.kr
- 2Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2450393
- DOI: http://doi.org/10.4070/kcj.2018.0379
Abstract
- BACKGROUND AND OBJECTIVES
Angiotensin II (Ang II) has been suggested to accelerate vascular senescence, however the molecular mechanism(s) remain unknown.
METHODS
We cultured human coronary artery smooth muscle cells (hCSMCs) and treated Ang II and/or fimasartan. Or we transfected adenoviral vectors expressing CYR61 (Ad-CYR61) or antisense CYR61 (Ad-As-CYR61). Cellular senescence was evaluated senescence-associated β-galactosidase (SA-β-gal) assay. The molecular mechanisms were investigated real-time PCR and western blots.
RESULTS
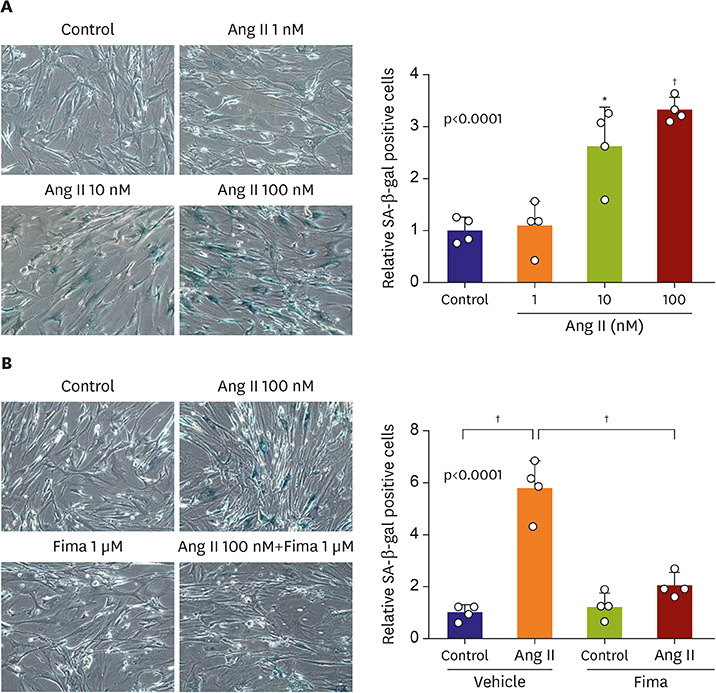
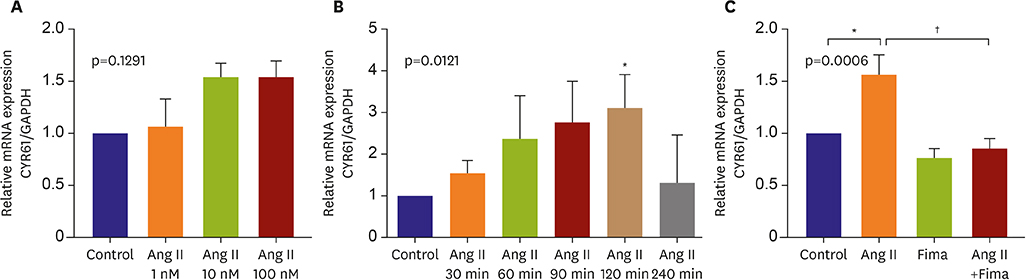
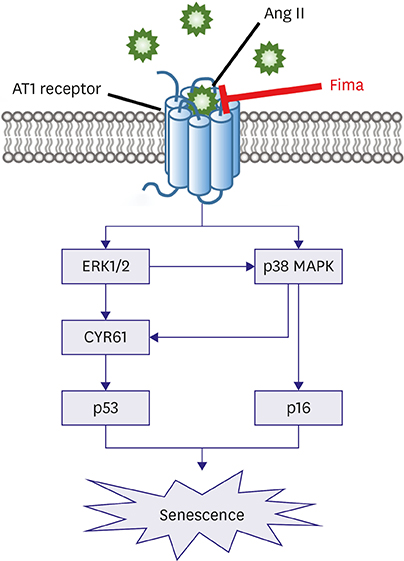
SA-β-gal-positive cells significantly increased in Ang II-treated hCSMCs (5.77±1.43-fold compared with the control). The effect of Ang II was significantly attenuated by pretreatment with the Ang II type 1 receptor blocker, fimasartan (2.00±0.92-fold). The expression of both p53 and p16 senescence regulators was significantly increased by Ang II (p53: 1.39±0.17, p16: 1.19±0.10-fold vs. the control), and inhibited by fimasartan. Cysteine-rich angiogenic protein 61 (CYR61) was rapidly induced by Ang II. Compared with the control, Ad-CYR61-transfected hCSMCs showed significantly increased SA-β-gal-positive cells (3.47±0.65-fold). Upon transfecting Ad-AS-CYR61, Ang II-induced senescence (3.74±0.23-fold) was significantly decreased (1.77±0.60-fold). p53 expression by Ang II was significantly attenuated by Ad-AS-CYR61, whereas p16 expression was not regulated. Ang II activated ERK1/2 and p38 MAPK, which was significantly blocked by fimasartan. ERK and p38 inhibition both regulated Ang II-induced CYR61 expression. However, p53 expression was only regulated by ERK1/2, whereas p16 expression was only attenuated by p38 MAPK.
CONCLUSIONS
Ang II induced vascular senescence by the ERK/p38 MAPK-CYR61 pathway and ARB, fimasartan, protected against Ang II-induced vascular senescence.
Keyword
MeSH Terms
-
Aging
Angiotensin II Type 1 Receptor Blockers
Angiotensin II*
Angiotensins*
Blotting, Western
Cell Aging*
Coronary Vessels
Humans
Muscle, Smooth, Vascular*
Myocytes, Smooth Muscle
p38 Mitogen-Activated Protein Kinases
Real-Time Polymerase Chain Reaction
Receptor, Angiotensin, Type 1*
Angiotensin II
Angiotensin II Type 1 Receptor Blockers
Angiotensins
Receptor, Angiotensin, Type 1
p38 Mitogen-Activated Protein Kinases
Figure
Cited by 2 articles
-
Renin-Angiotensin System Blockade in Acute Myocardial Infarction: Is There a Winner?
Hak Seung Lee, Jeehoon Kang
Korean Circ J. 2020;50(11):995-997. doi: 10.4070/kcj.2020.0398.Role of Inflammation in Arterial Calcification
Hae-Young Lee, Soyeon Lim, Sungha Park
Korean Circ J. 2021;51(2):114-125. doi: 10.4070/kcj.2020.0517.
Reference
-
1. Lee SE, Lee HY, Cho HJ, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF). Korean Circ J. 2017; 47:341–353.2. Norheim OF, Jha P, Admasu K, et al. Avoiding 40% of the premature deaths in each country, 2010–30: review of national mortality trends to help quantify the UN sustainable development goal for health. Lancet. 2015; 385:239–252.3. Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007; 8:729–740.4. Nilsson PM, Boutouyrie P, Cunha P, et al. Early vascular ageing in translation: from laboratory investigations to clinical applications in cardiovascular prevention. J Hypertens. 2013; 31:1517–1526.5. Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990; 345:458–460.6. Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995; 92:9363–9367.7. Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997; 88:593–602.8. Zhou MS, Hernandez Schulman I, Pagano PJ, Jaimes EA, Raij L. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension. 2006; 47:81–86.9. Anderson S, Jung FF, Ingelfinger JR. Renal renin-angiotensin system in diabetes: functional, immunohistochemical, and molecular biological correlations. Am J Physiol. 1993; 265:F477–86.10. Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991; 83:1849–1865.11. Kranzhöfer R, Schmidt J, Pfeiffer CA, Hagl S, Libby P, Kübler W. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999; 19:1623–1629.12. Shim KY, Eom YW, Kim MY, Kang SH, Baik SK. Role of the renin-angiotensin system in hepatic fibrosis and portal hypertension. Korean J Intern Med. 2018; 33:453–461.13. Kunieda T, Minamino T, Nishi J, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006; 114:953–960.14. de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: a strategy to slow ageing by protecting mitochondria? Cardiovasc Res. 2011; 89:31–40.15. Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007; 293:H1351–8.16. Li YW, Li YM, Hon Y, et al. AT1 receptor modulator attenuates the hypercholesterolemia-induced impairment of the myocardial ischemic post-conditioning benefits. Korean Circ J. 2017; 47:182–192.17. Jo YI, Na HY, Moon JY, et al. Effect of low-dose valsartan on proteinuria in normotensive immunoglobulin A nephropathy with minimal proteinuria: a randomized trial. Korean J Intern Med. 2016; 31:335–343.18. Lee JH, Bae MH, Yang DH, et al. Angiotensin II type 1 receptor blockers as a first choice in patients with acute myocardial infarction. Korean J Intern Med. 2016; 31:267–276.19. Kim TW, Yoo BW, Lee JK, et al. Synthesis and antihypertensive activity of pyrimidin-4(3H)-one derivatives as losartan analogue for new angiotensin II receptor type 1 (AT1) antagonists. Bioorg Med Chem Lett. 2012; 22:1649–1654.20. Sim DS, Jeong MH, Song HC, et al. Cardioprotective effect of fimasartan, a new angiotensin receptor blocker, in a porcine model of acute myocardial infarction. J Korean Med Sci. 2015; 30:34–43.21. Yang XL, Kim CK, Kim TJ, et al. Anti-inflammatory effects of fimasartan via Akt, ERK, and NFκB pathways on astrocytes stimulated by hemolysate. Inflamm Res. 2016; 65:115–123.22. Hilfiker A, Hilfiker-Kleiner D, Fuchs M, et al. Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II. Circulation. 2002; 106:254–260.23. Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999; 248:44–57.24. O'Brien TP, Yang GP, Sanders L, Lau LF. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990; 10:3569–3577.25. Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1998; 95:6355–6360.26. Lee HY, Chung JW, Youn SW, et al. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2007; 100:372–380.27. Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010; 12:676–685.28. Min LJ, Mogi M, Iwai M, Horiuchi M. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev. 2009; 8:113–121.29. Lee SJ, Park SH. Arterial ageing. Korean Circ J. 2013; 43:73–79.30. Tsai IC, Pan ZC, Cheng HP, Liu CH, Lin BT, Jiang MJ. Reactive oxygen species derived from NADPH oxidase 1 and mitochondria mediate angiotensin II-induced smooth muscle cell senescence. J Mol Cell Cardiol. 2016; 98:18–27.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Correction of Figures in the Article “Angiotensin II Type 1 Receptor Blocker, Fimasartan, Reduces Vascular Smooth Muscle Cell Senescence by Inhibiting the CYR61 Signaling Pathway”
- Downregulation of Angiotensin II-Induced 12-Lipoxygenase Expression and Cell Proliferation in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats by CCL5
- Sulfatase 1 mediates the inhibitory effect of angiotensin II type 2 receptor inhibitor on angiotensin II-induced hypertensive mediator expression and proliferation in vascular smooth muscle cells from spontaneously hypertensive rats
- Pitavastatin Regulates Ang II Induced Proliferation and Migration via IGFBP-5 in VSMC
- Losartan Inhibits Vascular Smooth Muscle Cell Proliferation through Activation of AMP-Activated Protein Kinase