Transl Clin Pharmacol.
2017 Mar;25(1):43-51. 10.12793/tcp.2017.25.1.43.
Decreased potency of fimasartan in liver cirrhosis was quantified using mixed-effects analysis
- Affiliations
-
- 1Department of Clinical Pharmacology, Severance Hospital, Yonsei University College of Medicine, Seoul 03722, Korea.
- 2Qfitter Inc, Seoul, Korea.
- 3Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. yimds@catholic.ac.kr
- 4Department of Pediatrics, Yonsei University College of Medicine, Seoul 03722, Korea.
- 5Clinical Trial Center, Kyungpook National University Hospital, Daegu 41944, Korea.
- KMID: 2411418
- DOI: http://doi.org/10.12793/tcp.2017.25.1.43
Abstract
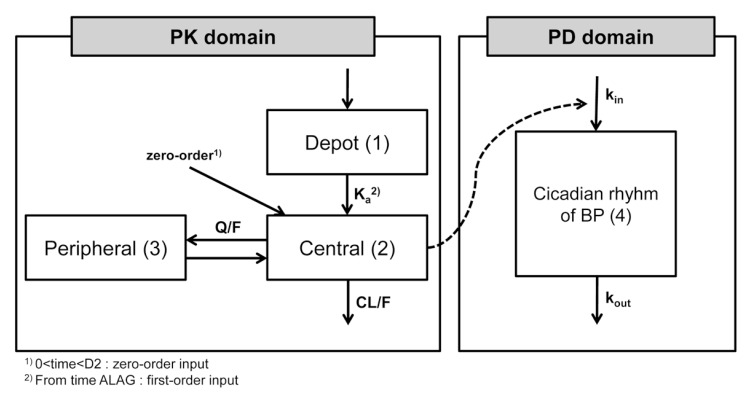
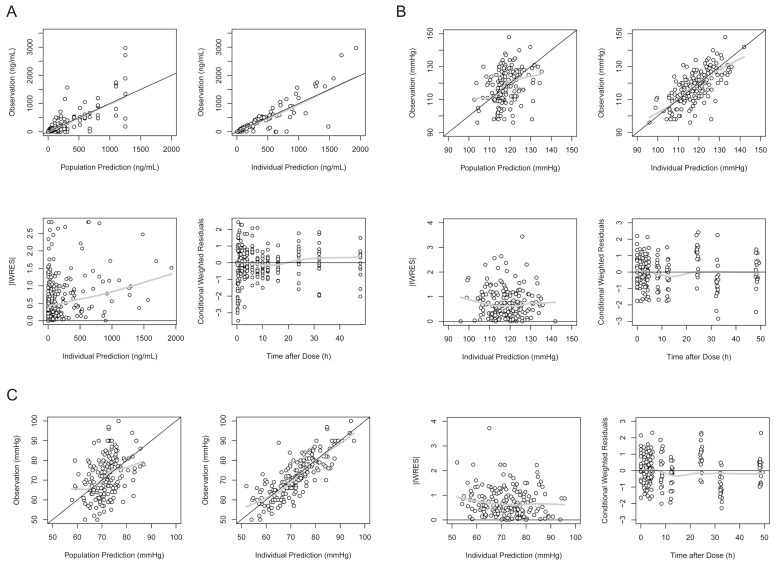
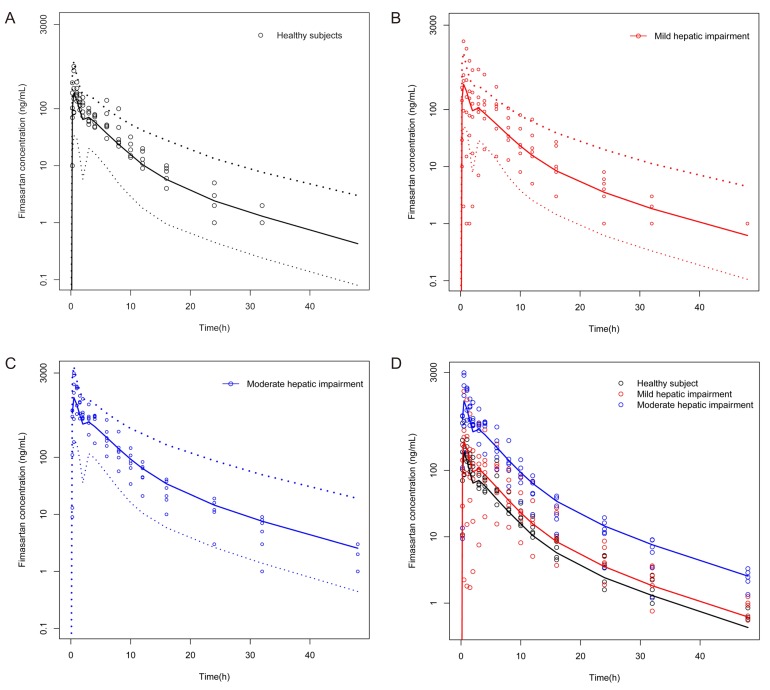
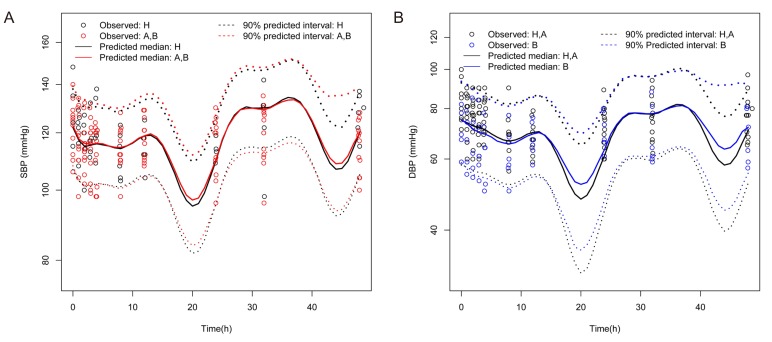
- Fimasartan is a nonpeptide angiotensin II receptor blocker. In a previous study that compared the pharmacokinetics (PK) of fimasartan between patients with hepatic impairment (cirrhosis) and healthy subjects, the exposure to fimasartan was found to be higher in patients, but the decrease of blood pressure (BP) was not clinically significant in those with moderate hepatic impairment. The aims of this study were to develop a population PK-pharmacodynamic (PD) model of fimasartan and to evaluate the effect of hepatic function on BP reduction by fimasartan using previously published data. A 2-compartment linear model with mixed zero-order absorption followed by first-order absorption with a lag time adequately described fimasartan PK, and the effect of fimasartan on BP changes was well explained by the inhibitory sigmoid function in the turnover PK-PD model overlaid with a model of circadian rhythm (NONMEM version 7.2). According to our PD model, the lower BP responses in hepatic impairment were the result of the increased fimasartan ECâ‚…â‚€ in patients, rather than from a saturation of effect. This is congruent with the reported pathophysiological change of increased plasma ACE and renin activity in hepatic cirrhosis.
MeSH Terms
Figure
Reference
-
1. Kim JH, Cho YZ, Na JH, Kim HS, Kang HW, Baik SK, et al. Effects of candesartan and propranolol combination therapy versus propranolol monotherapy in reducing portal hypertension. Clin Mol Hepatol. 2014; 20:376–383. DOI: 10.3350/cmh.2014.20.4.376. PMID: 25548744.
Article2. Shin BS, Kim TH, Paik SH, Chi YH, Lee JH, Tan HK, et al. Simultaneous determination of fimasartan, a novel antihypertensive agent, and its active metabolite in rat plasma by liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2011; 25:1208–1214. DOI: 10.1002/bmc.1592. PMID: 21268050.
Article3. Chi YH, Lee H, Paik SH, Lee JH, Yoo BW, Kim JH, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of fimasartan following single and repeated oral administration in the fasted and fed states in healthy subjects. Am J Cardiovasc Drugs. 2011; 11:335–346. DOI: 10.2165/11593840-000000000-00000. PMID: 21910510.
Article4. Ghim JL, Paik SH, Hasanuzzaman M, Chi YH, Choi HK, Kim DH, et al. Absolute bioavailability and pharmacokinetics of the angiotensin II receptor antagonist fimasartan in healthy subjects. J Clin Pharmacol. 2016; 56:576–580. DOI: 10.1002/jcph.618. PMID: 26272450.
Article5. Shin KH, Kim TE, Kim SE, Lee MG, Song IS, Yoon SH, et al. The effect of the newly developed angiotensin receptor II antagonist fimasartan on the pharmacokinetics of atorvastatin in relation to OATP1B1 in healthy male volunteers. J Cardiovasc Pharmacol. 2011; 58:492–499. DOI: 10.1097/FJC.0b013e31822b9092. PMID: 21765368.
Article6. Jeong ES, Kim YW, Kim HJ, Shin HJ, Shin JG, Kim KH, et al. Glucuronidation of fimasartan, a new angiotensin receptor antagonist, is mainly mediated by UGT1A3. Xenobiotica. 2015; 45:10–18. DOI: 10.3109/00498254.2014.942810. PMID: 25034008.
Article7. Kim TH, Shin S, Bashir M, Chi YH, Paik SH, Lee JH, et al. Pharmacokinetics and metabolite profiling of fimasartan, a novel antihypertensive agent, in rats. Xenobiotica. 2014; 44:913–925. DOI: 10.3109/00498254.2014.915359. PMID: 24786684.
Article8. Kim CO, Lee HW, Oh ES, Seong SJ, Kim DY, Lee J, et al. Influence of hepatic dysfunction on the pharmacokinetics and safety of fimasartan. J Cardiovasc Pharmacol. 2013; 62:524–529. DOI: 10.1097/FJC.0000000000000010. PMID: 24084213.
Article9. Lee J, Han S, Jeon S, Hong T, Yim DS. Pharmacokinetic-pharmacodynamic model of fimasartan applied to predict the influence of a high fat diet on its blood pressure-lowering effect in healthy subjects. Eur J Clin Pharmacol. 2013; 69:11–20. DOI: 10.1007/s00228-012-1297-3.
Article10. Kim JW, Yi S, Kim TE, Lim KS, Yoon SH, Cho JY, et al. Increased systemic exposure of fimasartan, an angiotensin II receptor antagonist, by ketoconazole and rifampicin. J Clin Pharmacol. 2013; 53:75–81. DOI: 10.1177/0091270011433328. PMID: 23400746.
Article11. Susla GM, Atkinson AJ Jr. Effect of Liver Disease on Pharmacokinetics. Principles of Clinical Pharmacology. 2nd Edition. San Diego: Londong: Boston: New York: Sydney: Tokyo: Toronto: Academic Press;2006.12. Kim TH, Shin S, Landersdorfer CB, Chi YH, Paik SH, Myung J, et al. Population pharmacokinetic modeling of the enterohepatic recirculation of fimasartan in rats, dogs, and humans. AAPS J. 2015; 17:1210–1223. DOI: 10.1208/s12248-015-9764-2. PMID: 25990964.
Article13. Lee H, Jang IJ, Yu KS, Choi J, Oh BH. A population pharmacokinetic pnalysis of pimasartan, a selective angiotensin II receptor antagonist, in healthy caucasian subjects and korean patients with hypertension. Clin Pharmacol Drug Dev. 2013; 2:162–172. DOI: 10.1002/cpdd.10. PMID: 27121670.14. Lee HW, Lim MS, Seong SJ, Lee J, Park J, Seo JJ, et al. Effect of age on the pharmacokinetics of fimasartan (BR-A-657). Expert Opin Drug Metab Toxicol. 2011; 7:1337–1344. DOI: 10.1517/17425255.2011.618835. PMID: 21950382.
Article15. van Rijn-Bikker PC, Ackaert O, Snelder N, van Hest RM, Ploeger BA, Koopmans RP, et al. Pharmacokinetic-pharmacodynamic modeling of the antihypertensive effect of eprosartan in Black and White hypertensive patients. Clin Pharmacokinet. 2013; 52:793–803. DOI: 10.1007/s40262-013-0073-6. PMID: 23696281.
Article16. Gross V, Treher E, Haag K, Neis W, Wiegand U, Schölmerich J. Angiotensin-converting enzyme (ACE)-inhibition in cirrhosis. Pharmacokinetics and dynamics of the ACE-inhibitor cilazapril (Ro 31-2848). J Hepatol. 1993; 17:40–47. PMID: 8445218.17. Tandon P, Abraldes JG, Berzigotti A, Garcia-Pagan JC, Bosch J. Renin-angiotensin-aldosterone inhibitors in the reduction of portal pressure: a systematic review and meta-analysis. J Hepatol. 2010; 53:273–282. DOI: 10.1016/j.jhep.2010.03.013. PMID: 20570385.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacodynamic Changes of Rocuronium during Induced Liver Cirrhosis Using Carbon Tetrachloride Intoxication in Rabbits
- Clinical Observation of Nail Changes in Systemic Diseases: II. Liver Cirrhosis
- Management of Liver Cirrhosis
- Fimasartan Ameliorates Deteriorations in Glucose Metabolism in a High Glucose State by Regulating Skeletal Muscle and Liver Cells
- The effects of amino acid infusion on liver regeneration after partial hepatectomy in the rat with liver cirrhosis