Immune Netw.
2012 Oct;12(5):207-212. 10.4110/in.2012.12.5.207.
T Cell Immunoglobulin Mucin Domain (TIM)-3 Promoter Activity in a Human Mast Cell Line
- Affiliations
-
- 1Department of Microbiology, Ajou University School of Medicine, Suwon 442-749, Korea. sinsun@ajou.ac.kr
- 2Graduate Program of Molecular Medicine, Ajou University School of Medicine, Suwon 442-749, Korea.
- KMID: 2150750
- DOI: http://doi.org/10.4110/in.2012.12.5.207
Abstract
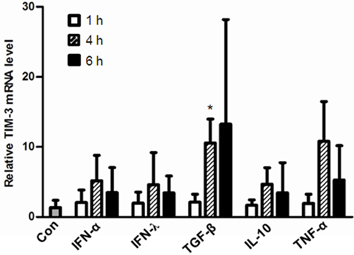
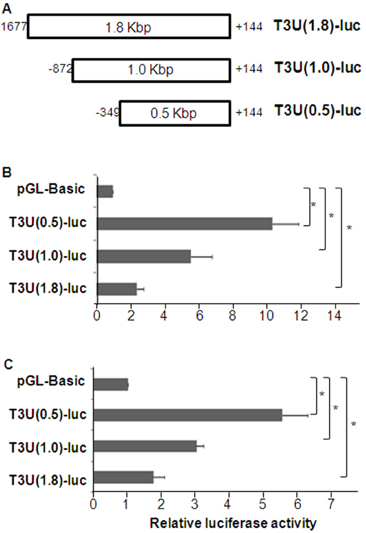
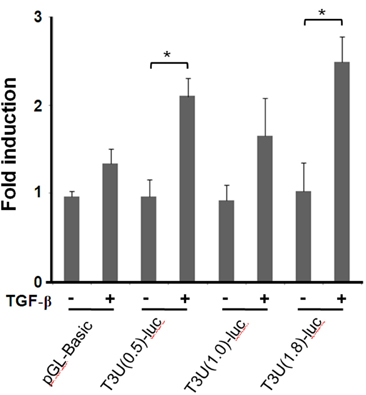
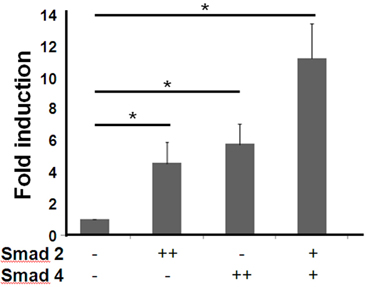
- T cell immunoglobulin mucin domain (TIM)-3 is an immunomodulatory molecule and upregulated in T cells by several cytokines. TIM-3 also influences mast cell function but its transcriptional regulation in mast cells has not been clarified. Therefore, we examined the transcript level and the promoter activity of TIM-3 in mast cells. The TIM-3 transcript level was assessed by real-time RT-PCR and promoter activity by luciferase reporter assay. TIM-3 mRNA levels were increased in HMC-1, a human mast cell line by TGF-beta1 stimulation but not by stimulation with interferon (IFN)-alpha, IFN-lambda, TNF-alpha, or IL-10. TIM-3 promoter -349~+144 bp region relative to the transcription start site was crucial for the basal and TGF-beta1-induced TIM-3 promoter activities in HMC-1 cells. TIM-3 promoter activity was increased by overexpression of Smad2 and Smad4, downstream molecules of TGF-beta1 signaling. Our results localize TIM-3 promoter activity to the region spanning -349 to +144 bp in resting and TGF-beta1 stimulated mast cells.
MeSH Terms
-
Cytokines
Humans
Immunoglobulins
Interferons
Interleukin-10
Luciferases
Mast Cells
Mucins
RNA, Messenger
T-Lymphocytes
Transcription Initiation Site
Transforming Growth Factor beta1
Tumor Necrosis Factor-alpha
Cytokines
Immunoglobulins
Interferons
Interleukin-10
Luciferases
Mucins
RNA, Messenger
Transforming Growth Factor beta1
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011. 32:345–349.
Article2. Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010. 207:2187–2194.
Article3. Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, Blazar BR. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011. 117:4501–4510.
Article4. Lee MJ, Woo MY, Chwae YJ, Kwon MH, Kim K, Park S. Down-regulation of interleukin-2 production by CD4(+) T cells expressing TIM-3 through suppression of NFAT dephosphorylation and AP-1 transcription. Immunobiology. 2012. 217:986–995.
Article5. Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, Hirashima M, Uede T, Takaoka A, Yagita H, Jinushi M. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012. 13:832–842.
Article6. Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009. 113:3821–3830.
Article7. Nakae S, Iikura M, Suto H, Akiba H, Umetsu DT, Dekruyff RH, Saito H, Galli SJ. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007. 110:2565–2568.
Article8. Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007. 7:93–104.
Article9. Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996. 381:77–80.
Article10. Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002. 297:1689–1692.
Article11. Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, Strom TB, Zheng XX, Noelle RJ. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006. 442:997–1002.
Article12. Wiener Z, Kohalmi B, Pocza P, Jeager J, Tolgyesi G, Toth S, Gorbe E, Papp Z, Falus A. TIM-3 is expressed in melanoma cells and is upregulated in TGF-beta stimulated mast cells. J Invest Dermatol. 2007. 127:906–914.
Article13. Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007. 8:970–982.
Article14. Conidi A, Cazzola S, Beets K, Coddens K, Collart C, Cornelis F, Cox L, Joke D, Dobreva MP, Dries R, Esguerra C, Francis A, Ibrahimi A, Kroes R, Lesage F, Maas E, Moya I, Pereira PN, Stappers E, Stryjewska A, van den Berghe V, Vermeire L, Verstappen G, Seuntjens E, Umans L, Zwijsen A, Huylebroeck D. Few Smad proteins and many Smad-interacting proteins yield multiple functions and action modes in TGFβ/BMP signaling in vivo. Cytokine Growth Factor Rev. 2011. 22:287–300.
Article15. Soond SM, Chantry A. Selective targeting of activating and inhibitory Smads by distinct WWP2 ubiquitin ligase isoforms differentially modulates TGFβ signalling and EMT. Oncogene. 2011. 30:2451–2462.
Article16. Broide DH, Wasserman SI, Alvaro-Gracia J, Zvaifler NJ, Firestein GS. Transforming growth factor-beta 1 selectively inhibits IL-3-dependent mast cell proliferation without affecting mast cell function or differentiation. J Immunol. 1989. 143:1591–1597.17. Macey MR, Sturgill JL, Morales JK, Falanga YT, Morales J, Norton SK, Yerram N, Shim H, Fernando J, Gifillan AM, Gomez G, Schwartz L, Oskeritzian C, Spiegel S, Conrad D, Ryan JJ. IL-4 and TGF-beta 1 counterbalance one another while regulating mast cell homeostasis. J Immunol. 2010. 184:4688–4695.
Article18. Miller HR, Wright SH, Knight PA, Thornton EM. A novel function for transforming growth factor-beta1: upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific beta-chymase, mouse mast cell protease-1. Blood. 1999. 93:3473–3486.
Article19. Mortaz E, Givi ME, Da Silva CA, Folkerts G, Redegeld FA. A relation between TGF-β and mast cell tryptase in experimental emphysema models. Biochim Biophys Acta. 2012. 1822:1154–1160.
Article20. Ganeshan K, Bryce PJ. Regulatory T cells enhance mast cell production of IL-6 via surface-bound TGF-β. J Immunol. 2012. 188:594–603.
Article21. Yoon SJ, Lee MJ, Shin DC, Kim JS, Chwae YJ, Kwon MH, Kim K, Park S. Activation of mitogen activated protein kinase-Erk kinase (MEK) increases T cell immunoglobulin mucin domain-3 (TIM-3) transcription in human T lymphocytes and a human mast cell line. Mol Immunol. 2011. 48:1778–1783.
Article22. Zhang J, Daley D, Akhabir L, Stefanowicz D, Chan-Yeung M, Becker AB, Laprise C, Paré PD, Sandford AJ. Lack of association of TIM3 polymorphisms and allergic phenotypes. BMC Med Genet. 2009. 10:62.23. Anderson AC, Lord GM, Dardalhon V, Lee DH, Sabatos-Peyton CA, Glimcher LH, Kuchroo VK. T-bet, a Th1 transcription factor regulates the expression of Tim-3. Eur J Immunol. 2010. 40:859–866.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular variations in Th1-specific cell surface gene Tim-3
- The Binding Properties of Glycosylated and Non-Glycosylated Tim-3 Molecules on CD4+CD25+ T Cells
- T-Cell Dysfunction and Inhibitory Receptors in Hepatitis C Virus Infection
- Identification of CCL1 as a Gene Differentially Expressed in CD4+ T Cells Expressing TIM-3
- Induction and Expression of Chemokines and Their Receptors in Human Mast Cell Line ( HMC - 1 )