Immune Netw.
2009 Apr;9(2):58-63. 10.4110/in.2009.9.2.58.
The Binding Properties of Glycosylated and Non-Glycosylated Tim-3 Molecules on CD4+CD25+ T Cells
- Affiliations
-
- 1Department of Microbiology and Immunology, Ajou University School of Medicine, Suwon, Korea. sinsun@ajou.ac.kr
- KMID: 1474596
- DOI: http://doi.org/10.4110/in.2009.9.2.58
Abstract
- BACKGROUND
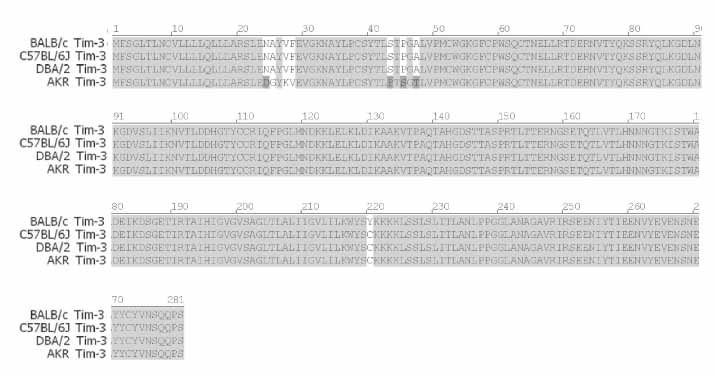
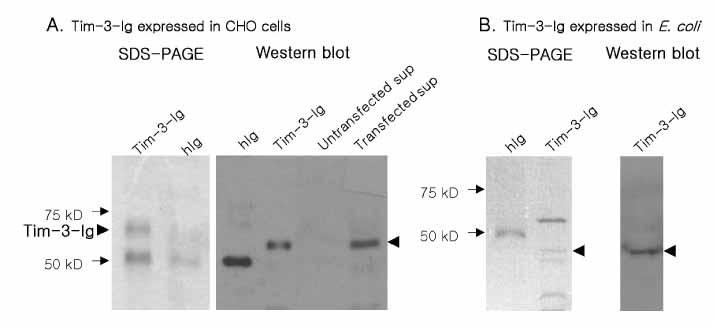
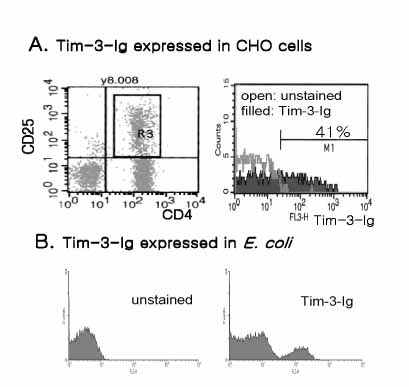
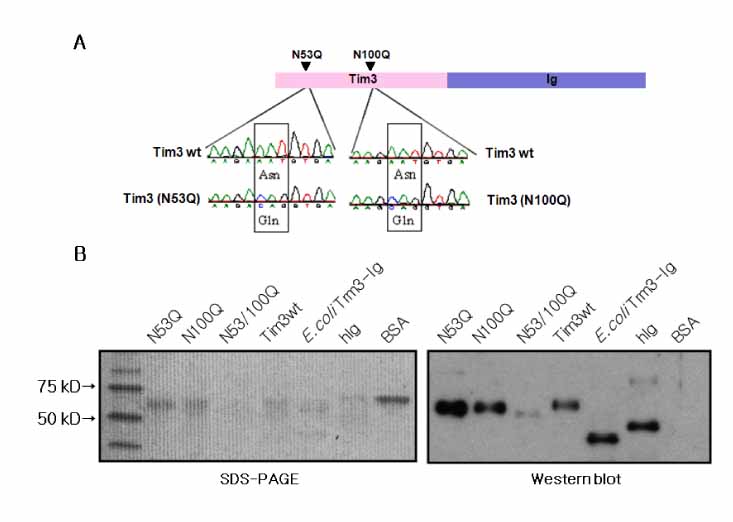
T cell immunoglobulin and mucin domain containing 3 protein (Tim-3) expressed on terminally differentiated Th1 cells plays a suppressive role in Th1-mediated immune responses. Recently, it has been shown that N-glycosylation affects the binding activity of the Tim-3-Ig fusion protein to its ligand, galectin-9, but the binding properties of non-glycosylated Tim-3 on CD4+CD25+ T cells has not been fully examined. In this study, we produced recombinant Tim-3-Ig fusion proteins in different cellular sources and its N-glycosylation mutant forms to evaluate their binding activities to CD4+CD25+ T cells. METHODS: We isolated and cloned Tim-3 cDNA from BALB/C mouse splenocytes. Then, we constructed a mammalian expression vector and a prokaryotic expression vector for the Tim-3-Ig fusion protein. Using a site directed mutagenesis method, plasmid vectors for Tim-3-Ig N-glycosylation mutant expression were produced. The recombinant protein was purified by protein A sepharose column chromatography. The binding activity of Tim-3-Ig fusion protein to CD4+CD25+ T cells was analyzed using flow cytometry. RESULTS: We found that the nonglycosylated Tim-3-Ig fusion proteins expressed in bacteria bound to CD4+CD25+ T cells similarly to the glycosylated Tim-3-Ig protein produced in CHO cells. Further, three N-glycosylation mutant forms (N53Q, N100Q, N53/100Q) of Tim-3-Ig showed similar binding activities to those of wild type glycosylated Tim-3-Ig. CONCLUSION: Our results suggest that N-glycosylation of Tim-3 may not affect its binding activity to ligands expressed on CD4+CD25+ T cells.
Keyword
MeSH Terms
-
Animals
Bacteria
CHO Cells
Chromatography
Clone Cells
Cricetinae
DNA, Complementary
Flow Cytometry
Immunoglobulins
Ligands
Mice
Mucins
Mutagenesis, Site-Directed
Plasmids
Proteins
Sepharose
Staphylococcal Protein A
T-Lymphocytes
Th1 Cells
DNA, Complementary
Immunoglobulins
Ligands
Mucins
Proteins
Sepharose
Staphylococcal Protein A
Figure
Reference
-
1. Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002. 415:536–541.
Article2. Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003. 4:1102–1110.
Article3. Wiener Z, Kohalmi B, Pocza P, Jeager J, Tolgyesi G, Toth S, Gorbe E, Papp Z, Falus A. TIM-3 is expressed in melanoma cells and is upregulated in TGF-beta stimulated mast cells. J Invest Dermatol. 2007. 127:906–914.
Article4. Kuchroo VK, Meyers JH, Umetsu DT, DeKruyff RH. TIM family of genes in immunity and tolerance. Adv Immunol. 2006. 91:227–249.
Article5. Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, Gutierrez-Ramos JC, Coyle AJ, Strom TB. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003. 4:1093–1101.
Article6. Frisancho-Kiss S, Nyland JF, Davis SE, Barrett MA, Gatewood SJ, Njoku DB, Cihakova D, Silbergeld EK, Rose NR, Fairweather D. Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J Immunol. 2006. 176:6411–6415.
Article7. Oikawa T, Kamimura Y, Akiba H, Yagita H, Okumura K, Takahashi H, Zeniya M, Tajiri H, Azuma M. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J Immunol. 2006. 177:4281–4287.
Article8. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005. 6:1245–1252.
Article9. Medzihradszky KF. Characterization of site-specific N-glycosylation. Methods Mol Biol. 2008. 446:293–316.
Article10. Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Fedorov A, Fedorov E, Zencheck WD, Lary JW, Cole JL, Deng H, Xiao H, Dilorenzo TP, Allison JP, Nathenson SG, Almo SC. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 2007. 26:311–321.
Article11. Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, Ito K, Takeshita K, Niki T, Saita N, Nishi N, Yamauchi A, Katoh S, Matsukawa A, Kuchroo V, Hirashima M. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008. 127:78–88.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Number of Circulating CD4+CD25+Foxp3+ Regulatory T Cells and CD4+CD25-Foxp3+ T Cells in Psoriasis
- Peripheral Generation of CD4+ CD25+ Foxp3+ Regulatory T Cells
- IL-4 Induces CD4+CD25+ Regulatory T Cells from CD4+CD25- T Cells in Peripheral Blood
- Identification of CCL1 as a Gene Differentially Expressed in CD4+ T Cells Expressing TIM-3
- Dynamic Frequency of Blood CD4+CD25+ Regulatory T Cells in Rats with Collagen-induced Arthritis