Immune Netw.
2010 Dec;10(6):198-205. 10.4110/in.2010.10.6.198.
Codelivery of IL-7 Augments Multigenic HCV DNA Vaccine-induced Antibody as well as Broad T Cell Responses in Cynomolgus Monkeys
- Affiliations
-
- 1Division of Molecular and Life Science, Integrative Bioscience and Biotechnology, WCU, Pohang University of Science and Technology (POSTECH), Pohang 790-784, Korea. ycsung@postech.ac.kr
- KMID: 2150675
- DOI: http://doi.org/10.4110/in.2010.10.6.198
Abstract
- BACKGROUND
A crucial limitation of DNA vaccines is its weak immunogenicity, especially in terms of eliciting antibody responses in non-human primates or humans; therefore, it is essential to enhance immune responses to vaccination for the development of successful DNA vaccines for humans.
METHODS
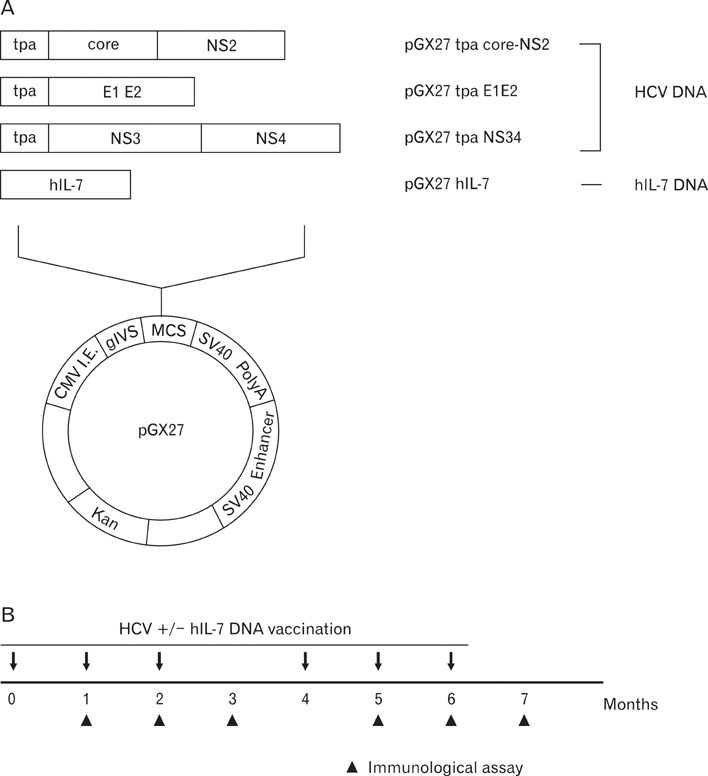
Here, we approached this issue by evaluating interleukin-7 (IL-7) as a genetic adjuvant in cynomolgus monkeys immunized with multigenic HCV DNA vaccine.
RESULTS
Codelivery of human IL-7 (hIL-7)-encoding DNA appeared to increase DNA vaccine-induced antibody responses specific for HCV E2 protein, which plays a critical role in protecting from HCV infection. HCV-specific T cell responses were also significantly enhanced by codelivery of hIL-7 DNA. Interestingly, the augmentation of T cell responses by codelivery of hIL-7 DNA was shown to be due to the enhancement of both the breadth and magnitude of immune responses against dominant and subdominant epitopes.
CONCLUSION
Taken together, these findings suggest that the hIL-7-expressing plasmid serves as a promising vaccine adjuvant capable of eliciting enhanced vaccine-induced antibody and broad T cell responses.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Herpes Zoster DNA Vaccines with IL-7 and IL-33 Molecular Adjuvants Elicit Protective T Cell Immunity
A Reum Kim, Junsik Park, Jong Hoon Kim, Jeong-Eun Kwak, Youngran Cho, Hyojin Lee, Moonsup Jeong, Su-Hyung Park, Eui-Cheol Shin
Immune Netw. 2018;18(5):. doi: 10.4110/in.2018.18.e38.
Reference
-
1. Kiyosawa K, Tanaka E, Sodeyama T, Furuta K, Usuda S, Yousuf M, Furuta S. Transition of antibody to hepatitis C virus from chronic hepatitis to hepatocellular carcinoma. Jpn J Cancer Res. 1990. 81:1089–1091.
Article2. Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999. 341:556–562.
Article3. Nascimbeni M, Mizukoshi E, Bosmann M, Major ME, Mihalik K, Rice CM, Feinstone SM, Rehermann B. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003. 77:4781–4793.
Article4. Lanford RE, Guerra B, Chavez D, Bigger C, Brasky KM, Wang XH, Ray SC, Thomas DL. Cross-genotype immunity to hepatitis C virus. J Virol. 2004. 78:1575–1581.
Article5. Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, Pezzanera M, Tafi R, Arcuri M, Fattori E, Lahm A, Luzzago A, Vitelli A, Colloca S, Cortese R, Nicosia A. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006. 12:190–197.
Article6. Youn JW, Park SH, Lavillette D, Cosset FL, Yang SH, Lee CG, Jin HT, Kim CM, Shata MT, Lee DH, Pfahler W, Prince AM, Sung YC. Sustained E2 antibody response correlates with reduced peak viremia after hepatitis C virus infection in the chimpanzee. Hepatology. 2005. 42:1429–1436.
Article7. Forns X, Payette PJ, Ma X, Satterfield W, Eder G, Mushahwar IK, Govindarajan S, Davis HL, Emerson SU, Purcell RH, Bukh J. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology. 2000. 32:618–625.
Article8. Pertmer TM, Roberts TR, Haynes JR. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996. 70:6119–6125.
Article9. Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci U S A. 1993. 90:11478–11482.
Article10. Choi SY, Suh YS, Cho JH, Jin HT, Chang J, Sung YC. Enhancement of DNA vaccine-induced immune responses by influenza virus NP gene. Immune Netw. 2009. 9:169–178.
Article11. Barouch DH, Letvin NL, Seder RA. The role of cytokine DNAs as vaccine adjuvants for optimizing cellular immune responses. Immunol Rev. 2004. 202:266–274.
Article12. Appasamy PM. Biological and clinical implications of interleukin-7 and lymphopoiesis. Cytokines Cell Mol Ther. 1999. 5:25–39.13. Faltynek CR, Wang S, Miller D, Young E, Tiberio L, Kross K, Kelley M, Kloszewski E. Administration of human recombinant IL-7 to normal and irradiated mice increases the numbers of lymphocytes and some immature cells of the myeloid lineage. J Immunol. 1992. 149:1276–1282.14. Komschlies KL, Gregorio TA, Gruys ME, Back TC, Faltynek CR, Wiltrout RH. Administration of recombinant human IL-7 to mice alters the composition of B-lineage cells and T cell subsets, enhances T cell function, and induces regression of established metastases. J Immunol. 1994. 152:5776–5784.15. Morrissey PJ, Goodwin RG, Nordan RP, Anderson D, Grabstein KH, Cosman D, Sims J, Lupton S, Acres B, Reed SG. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J Exp Med. 1989. 169:707–716.
Article16. Alderson MR, Sassenfeld HM, Widmer MB. Interleukin 7 enhances cytolytic T lymphocyte generation and induces lymphokine-activated killer cells from human peripheral blood. J Exp Med. 1990. 172:577–587.
Article17. Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000. 1:426–432.
Article18. Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001. 98:8732–8737.
Article19. Boise LH, Minn AJ, June CH, Lindsten T, Thompson CB. Growth factors can enhance lymphocyte survival without committing the cell to undergo cell division. Proc Natl Acad Sci U S A. 1995. 92:5491–5495.
Article20. Maeurer MJ, Trinder P, Hommel G, Walter W, Freitag K, Atkins D, Störkel S. Interleukin-7 or interleukin-15 enhances survival of Mycobacterium tuberculosis-infected mice. Infect Immun. 2000. 68:2962–2970.
Article21. Sin JI, Kim J, Pachuk C, Weiner DB. Interleukin 7 can enhance antigen-specific cytotoxic-T-lymphocyte and/or Th2-type immune responses in vivo. Clin Diagn Lab Immunol. 2000. 7:751–758.
Article22. Calarota SA, Dai A, Trocio JN, Weiner DB, Lori F, Lisziewicz J. IL-15 as memory T-cell adjuvant for topical HIV-1 DermaVir vaccine. Vaccine. 2008. 26:5188–5195.
Article23. Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005. 115:1177–1187.
Article24. Geiselhart LA, Humphries CA, Gregorio TA, Mou S, Subleski J, Komschlies KL. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J Immunol. 2001. 166:3019–3027.
Article25. Lee KJ, Suh YA, Cho YG, Cho YS, Ha GW, Chung KH, Hwang JH, Yun YD, Lee DS, Kim CM, Sung YC. Hepatitis C virus E2 protein purified from mammalian cells is frequently recognized by E2-specific antibodies in patient sera. J Biol Chem. 1997. 272:30040–30046.
Article26. Sheehan JJ, Tsirka SE. Fibrin-modifying serine proteases thrombin, tPA, and plasmin in ischemic stroke: a review. Glia. 2005. 50:340–350.
Article27. Youn JW, Park SH, Cho JH, Sung YC. Optimal induction of T-cell responses against hepatitis C virus E2 by antigen engineering in DNA immunization. J Virol. 2003. 77:11596–11602.
Article28. Park SH, Yang SH, Lee CG, Youn JW, Chang J, Sung YC. Efficient induction of T helper 1 CD4+ T-cell responses to hepatitis C virus core and E2 by a DNA prime-adenovirus boost. Vaccine. 2003. 21:4555–4564.
Article29. Park SH, Lee SR, Hyun BH, Kim BM, Sung YC. Codelivery of PEG-IFN-alpha inhibits HCV DNA vaccine-induced T cell responses but not humoral responses in African green monkeys. Vaccine. 2008. 26:3978–3983.
Article30. Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006. 24:657–679.
Article31. Sinha ML, Fry TJ, Fowler DH, Miller G, Mackall CL. Interleukin 7 worsens graft-versus-host disease. Blood. 2002. 100:2642–2649.
Article32. Milne CD, Paige CJ. IL-7: a key regulator of B lymphopoiesis. Semin Immunol. 2006. 18:20–30.
Article33. Morrissey PJ, Conlon P, Charrier K, Braddy S, Alpert A, Williams D, Namen AE, Mochizuki D. Administration of IL-7 to normal mice stimulates B-lymphopoiesis and peripheral lymphadenopathy. J Immunol. 1991. 147:561–568.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhancement of Antigen-specific Antibody and CD8(+) T Cell Responses by Codelivery of IL-12-encapsulated Microspheres in Protein and Peptide Vaccination
- Enhancement of DNA Vaccine-induced Immune Responses by Influenza Virus NP Gene
- Co-Immunization of Plasmid DNA Encoding IL-12 and IL-18 with Bacillus Calmette-Guerin Vaccine against Progressive Tuberculosis
- Perspectives of AIDS Vaccine Development T Cell-based Vaccine
- Efficient Induction of Th1-type Immune Responses to Hepatitis B Virus Antigens by DNA Prime-Adenovirus Boost