Immune Netw.
2009 Oct;9(5):169-178. 10.4110/in.2009.9.5.169.
Enhancement of DNA Vaccine-induced Immune Responses by Influenza Virus NP Gene
- Affiliations
-
- 1Research Institute, Genexine Co. Ltd., Pohang, Korea. ycsung@postech.ac.kr
- 2Garvan Institute of Medical Research, Sydney, New South Wales, Australia.
- 3Emory Vaccine Center and Department of Microbiology and Immunology, Emory University School of Medicine, Clifton Road, Atlanta, Georgia, USA.
- 4Division of Life and Pharmaceutical Sciences, and Center for Cell signaling & Drug Discovery Research, Ewha Womans University, Seoul, Korea.
- 5Laboratory of Cellular Immunology, Division of Molecular and Life Sciences, Pohang University of Science and Technology, Pohang, Korea.
- KMID: 1474578
- DOI: http://doi.org/10.4110/in.2009.9.5.169
Abstract
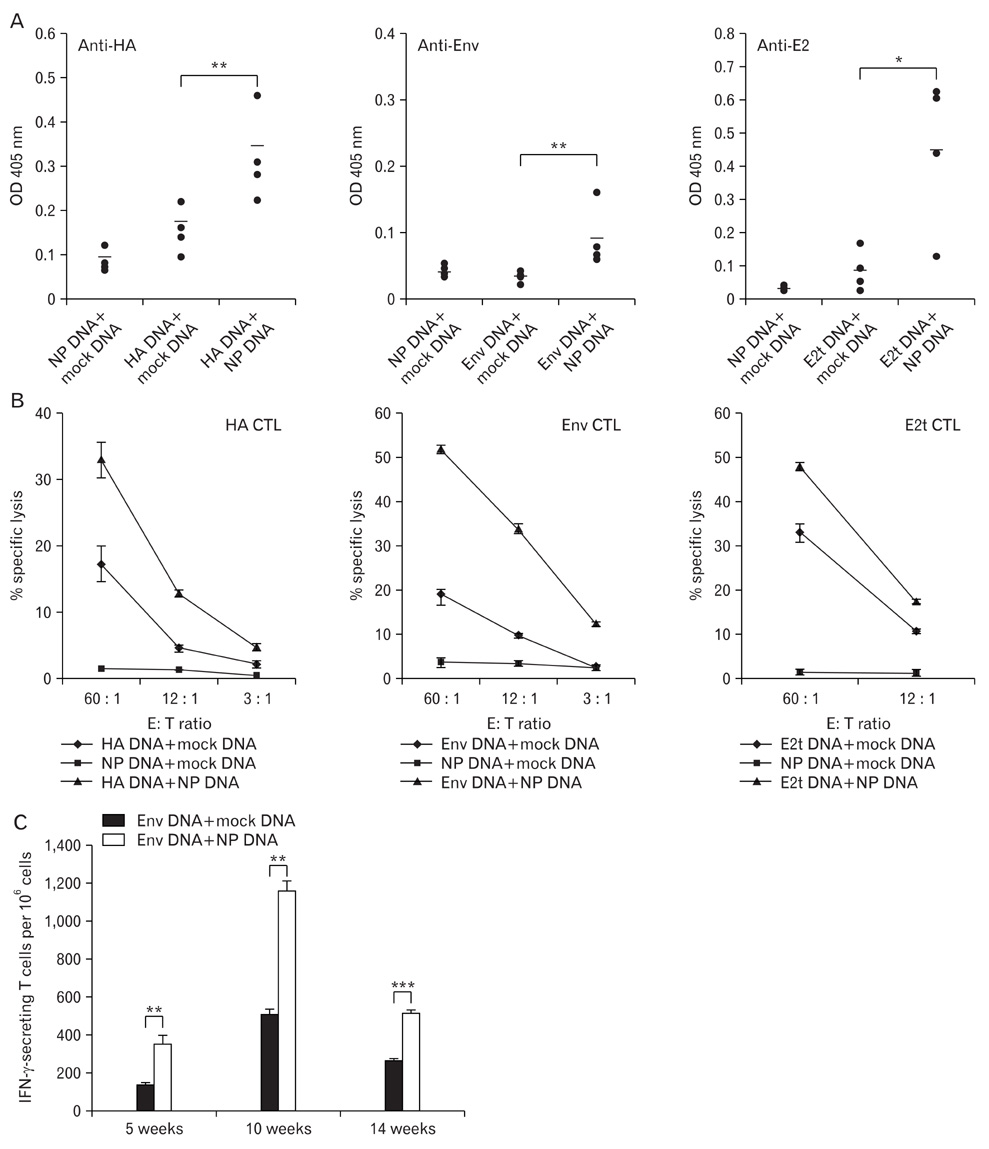
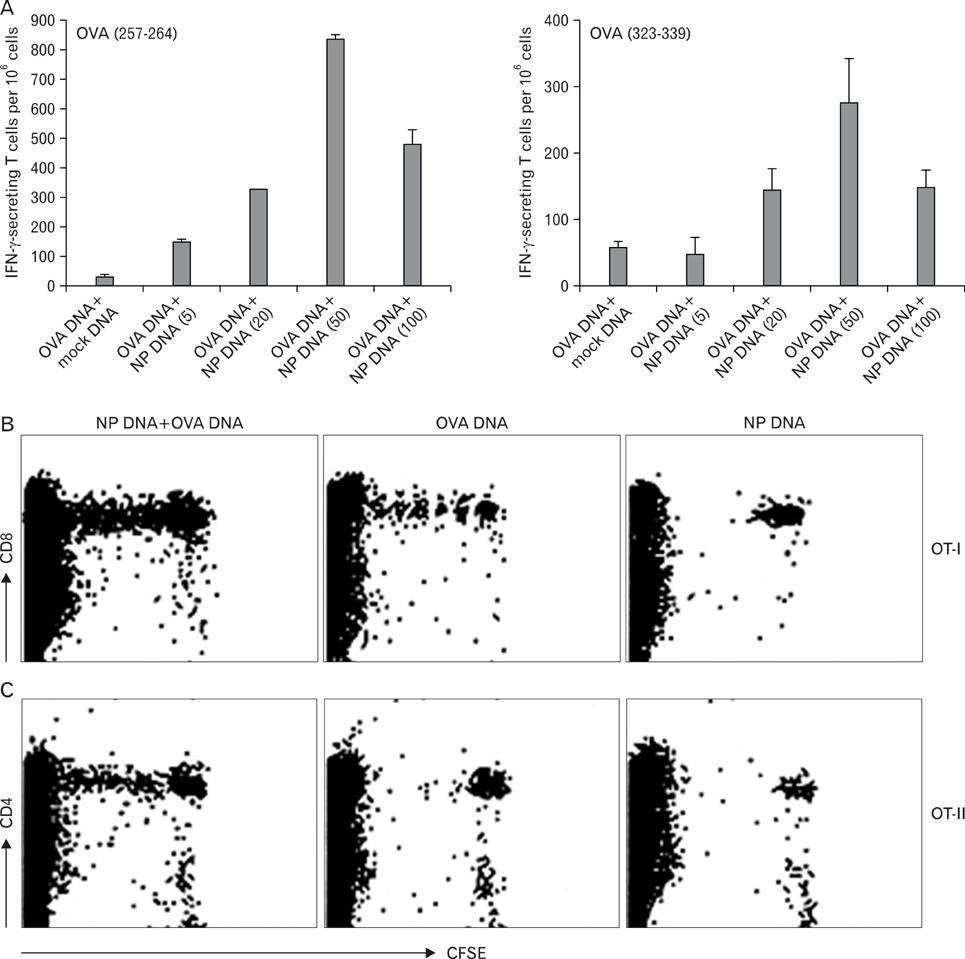
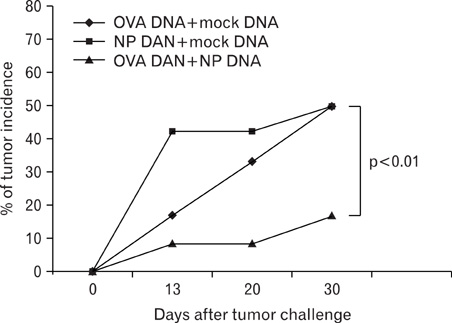
- DNA immunization induces B and T cell responses to various pathogens and tumors. However, these responses are known to be relatively weak and often transient. Thus, novel strategies are necessary for enhancing immune responses induced by DNA immunization. Here, we demonstrated that co-immunization of influenza virus nucleoprotein (NP) gene significantly enhances humoral and cell-mediated responses to codelivered antigens in mice. We also found that NP DNA coimmunization augments in vivo proliferation of adoptively transferred antigen-specific CD4 and CD8 T cells, which enhanced protective immunity against tumor challenge. Our results suggest that NP DNA can serve as a novel genetic adjuvant in cocktail DNA vaccination.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Codelivery of IL-7 Augments Multigenic HCV DNA Vaccine-induced Antibody as well as Broad T Cell Responses in Cynomolgus Monkeys
Su-Hyung Park, Mi-Young Song, Hyo Jung Nam, Se Jin Im, Young-Chul Sung
Immune Netw. 2010;10(6):198-205. doi: 10.4110/in.2010.10.6.198.
Reference
-
1. Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008. 9:776–788.
Article2. Liu MA. DNA vaccines: a review. J Intern Med. 2003. 253:402–410.
Article3. Beláková J, Horynová M, Krupka M, Weigl E, Raska M. DNA vaccines: are they still just a powerful tool for the future? Arch Immunol Ther Exp (Warsz). 2007. 55:387–398.4. Abdulhaqq SA, Weiner DB. DNA vaccines: developing new strategies to enhance immune responses. Immunol Res. 2008. 42:219–232.
Article5. Liu MA, Wahren B, Karlsson Hedestam GB. DNA vaccines: recent developments and future possibilities. Hum Gene Ther. 2006. 17:1051–1061.
Article6. Lee AH, Suh YS, Sung YC. DNA inoculations with HIV-1 recombinant genomes that express cytokine genes enhance HIV-1 specific immune responses. Vaccine. 1999. 17:473–479.
Article7. Suh YS, Ha SJ, Lee CH, Sin JI, Sung YC. Enhancement of VP1-specific immune responses and protection against EMCV-K challenge by co-delivery of IL-12 DNA with VP1 DNA vaccine. Vaccine. 2001. 19:1891–1898.
Article8. Yu DH, Li M, Hu XD, Cai H. A combined DNA vaccine enhances protective immunity against Mycobacterium tuberculosis and Brucella abortus in the presence of an IL-12 expression vector. Vaccine. 2007. 25:6744–6754.
Article9. Lee SW, Cho JH, Sung YC. Optimal induction of hepatitis C virus envelope-specific immunity by bicistronic plasmid DNA inoculation with the granulocyte-macrophage colony-stimulating factor gene. J Virol. 1998. 72:8430–8436.
Article10. Maksaereekul S, Dubie RA, Shen X, Kieu H, Dean GA, Sparger EE. Vaccination with vif-deleted feline immunodeficiency virus provirus, GM-CSF, and TNF-alpha plasmids preserves global CD4 T lymphocyte function after challenge with FIV. Vaccine. 2009. 27:3754–3765.
Article11. Gomez CE, Najera JL, Sanchez R, Jimenez V, Esteban M. Multimeric soluble CD40 ligand (sCD40L) efficiently enhances HIV specific cellular immune responses during DNA prime and boost with attenuated poxvirus vectors MVA and NYVAC expressing HIV antigens. Vaccine. 2009. 27:3165–3174.
Article12. Maue AC, Waters WR, Palmer MV, Whipple DL, Minion FC, Brown WC, Estes DM. CD80 and CD86, but not CD154, augment DNA vaccine-induced protection in experimental bovine tuberculosis. Vaccine. 2004. 23:769–779.
Article13. Stone GW, Barzee S, Snarsky V, Kee K, Spina CA, Yu XF, Kornbluth RS. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. J Virol. 2006. 80:1762–1772.
Article14. Salgaller ML, Lodge PA. Use of cellular and cytokine adjuvants in the immunotherapy of cancer. J Surg Oncol. 1998. 68:122–138.
Article15. Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002. 415:331–335.
Article16. Chaudhry UI, Kingham TP, Plitas G, Katz SC, Raab JR, DeMatteo RP. Combined stimulation with interleukin-18 and CpG induces murine natural killer dendritic cells to produce IFN-gamma and inhibit tumor growth. Cancer Res. 2006. 66:10497–10504.
Article17. Paget C, Bialecki E, Fontaine J, Vendeville C, Mallevaey T, Faveeuw C, Trottein F. Role of invariant NK T lymphocytes in immune responses to CpG oligodeoxynucleotides. J Immunol. 2009. 182:1846–1853.
Article18. Kojima Y, Xin KQ, Ooki T, Hamajima K, Oikawa T, Shinoda K, Ozaki T, Hoshino Y, Jounai N, Nakazawa M, Klinman D, Okuda K. Adjuvant effect of multi-CpG motifs on an HIV-1 DNA vaccine. Vaccine. 2002. 20:2857–2865.
Article19. Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen MD, Silverman GJ, Lotz M, Carson DA, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996. 273:352–354.
Article20. Seaman MS, Xu L, Beaudry K, Martin KL, Beddall MH, Miura A, Sambor A, Chakrabarti BK, Huang Y, Bailer R, Koup RA, Mascola JR, Nabel GJ, Letvin NL. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J Virol. 2005. 79:2956–2963.
Article21. Kjerrstrom A, Hinkula J, Engström G, Ovod V, Krohn K, Benthin R, Wahren B. Interactions of single and combined human immunodeficiency virus type 1 (HIV-1) DNA vaccines. Virology. 2001. 284:46–61.
Article22. Grifantini R, Finco O, Bartolini E, Draghi M, Del Giudice G, Kocken C, Thomas A, Abrignani S, Grandi G. Multi-plasmid DNA vaccination avoids antigenic competition and enhances immunogenicity of a poorly immunogenic plasmid. Eur J Immunol. 1998. 28:1225–1232.
Article23. Sin JI, Sung JH, Suh YS, Lee AH, Chung JH, Sung YC. Protective immunity against heterologous challenge with encephalomyocarditis virus by VP1 DNA vaccination: effect of coinjection with a granulocyte-macrophage colony stimulating factor gene. Vaccine. 1997. 15:1827–1833.
Article24. Song MK, Lee SW, Suh YS, Lee KJ, Sung YC. Enhancement of immunoglobulin G2a and cytotoxic T-lymphocyte responses by a booster immunization with recombinant hepatitis C virus E2 protein in E2 DNA-primed mice. J Virol. 2000. 74:2920–2925.
Article25. Power CA, Grand CL, Ismail N, Peters NC, Yurkowski DP, Bretscher PA. A valid ELISPOT assay for enumeration of ex vivo, antigen-specific, IFNgamma-producing T cells. J Immunol Methods. 1999. 227:99–107.
Article26. Kurts C, Miller JF, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998. 188:409–414.
Article27. Bot A, Bot S, Bona C. Enhanced protection against influenza virus of mice immunized as newborns with a mixture of plasmids expressing hemagglutinin and nucleoprotein. Vaccine. 1998. 16:1675–1682.
Article28. Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998. 8:177–187.
Article29. Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002. 2:251–262.
Article30. Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, Carbone FR, Heath WR. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001. 166:6099–6103.
Article31. Fu TM, Guan L, Friedman A, Schofield TL, Ulmer JB, Liu MA, Donnelly JJ. Dose dependence of CTL precursor frequency induced by a DNA vaccine and correlation with protective immunity against influenza virus challenge. J Immunol. 1999. 162:4163–4170.32. Justewicz DM, Morin MJ, Robinson HL, Webster RG. Antibody-forming cell response to virus challenge in mice immunized with DNA encoding the influenza virus hemagglutinin. J Virol. 1995. 69:7712–7717.
Article33. Fu TM, Friedman A, Ulmer JB, Liu MA, Donnelly JJ. Protective cellular immunity: cytotoxic T-lymphocyte responses against dominant and recessive epitopes of influenza virus nucleoprotein induced by DNA immunization. J Virol. 1997. 71:2715–2721.
Article34. Lee SW, Youn JW, Seong BL, Sung YC. IL-6 induces long-term protective immunity against a lethal challenge of influenza virus. Vaccine. 1999. 17:490–496.
Article35. Chen W, Antón LC, Bennink JR, Yewdell JW. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity. 2000. 12:83–93.
Article36. Potter P, Tourdot S, Blanchard T, Smith GL, Gould KG. Differential processing and presentation of the H-2D(b)-restricted epitope from two different strains of influenza virus nucleoprotein. J Gen Virol. 2001. 82:1069–1074.
Article37. Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999. 17:51–88.
Article38. Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002. 20:709–760.
Article39. Fomsgaard A, Nielsen HV, Bryder K, Nielsen C, Machuca R, Bruun L, Hansen J, Buns S. Improved humoral and cellular immune responses against the gp120 V3 loop of HIV-1 following genetic immunization with a chimeric DNA vaccine encoding the V3 inserted into the hepatitis B surface antigen. Scand J Immunol. 1998. 47(4):289–295.
Article40. Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996. 184(2):747–752.
Article41. Ramanathan S, Gagnon J, Ilangumaran S. Antigen-nonspecific activation of CD8 T lymphocytes by cytokines: relevance to immunity, autoimmunity, and cancer. Arch Immunol Ther Exp (Warsz). 2008. 56(5):311–323.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A “Prime and Deploy” Strategy for Universal Influenza Vaccine Targeting Nucleoprotein Induces Lung-Resident Memory CD8 T cells
- Vaccine Strategy That Enhances the Protective Efficacy of Systemic Immunization by Establishing LungResident Memory CD8 T Cells Against Influenza Infection
- Nucleoprotein vaccine induces cross-protective cytotoxic T lymphocytes against both lineages of influenza B virus
- Progress and hurdles in the development of influenza virus-like particle vaccines for veterinary use
- Codelivery of IL-7 Augments Multigenic HCV DNA Vaccine-induced Antibody as well as Broad T Cell Responses in Cynomolgus Monkeys