Allergy Asthma Immunol Res.
2015 Sep;7(5):489-496. 10.4168/aair.2015.7.5.489.
Human Rhinovirus-induced Proinflammatory Cytokine and Interferon-beta Responses in Nasal Epithelial Cells From Chronic Rhinosinusitis Patients
- Affiliations
-
- 1Department of Otolaryngology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jangyj@amc.seoul.kr
- 2Asan Institute for Life Science, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2147958
- DOI: http://doi.org/10.4168/aair.2015.7.5.489
Abstract
- PURPOSE
Asthma exacerbation from human rhinovirus (HRV) infection is associated with deficient antiviral interferon (IFN) secretion. Although chronic rhinosinusitis (CRS), an inflammatory upper airway disease, is closely linked to asthma, IFN-beta responses to HRV infections in human nasal epithelial cells (HNECs) from CRS patients remain to be studied. We evaluated inflammatory and antiviral responses to HRV infection in HNECs from CRS patients.
METHODS
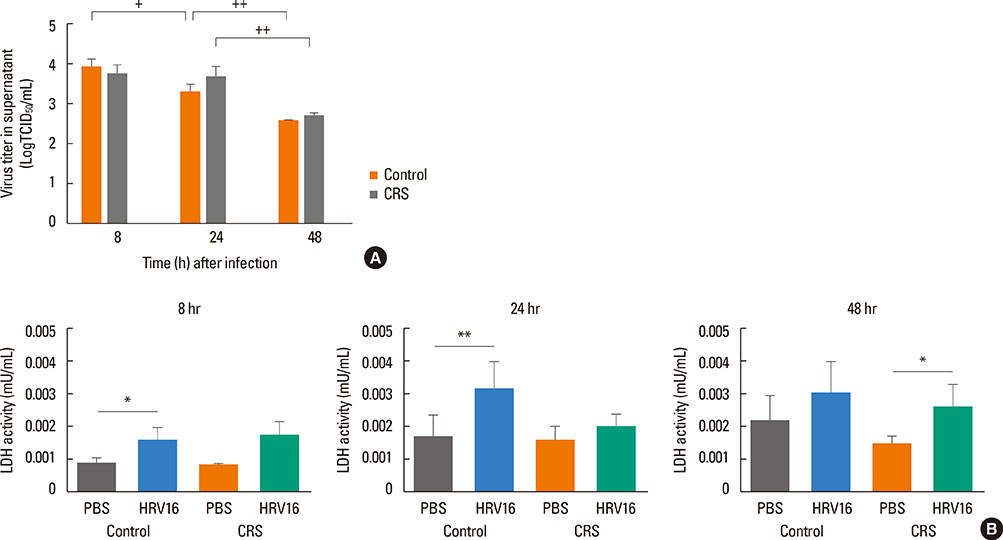
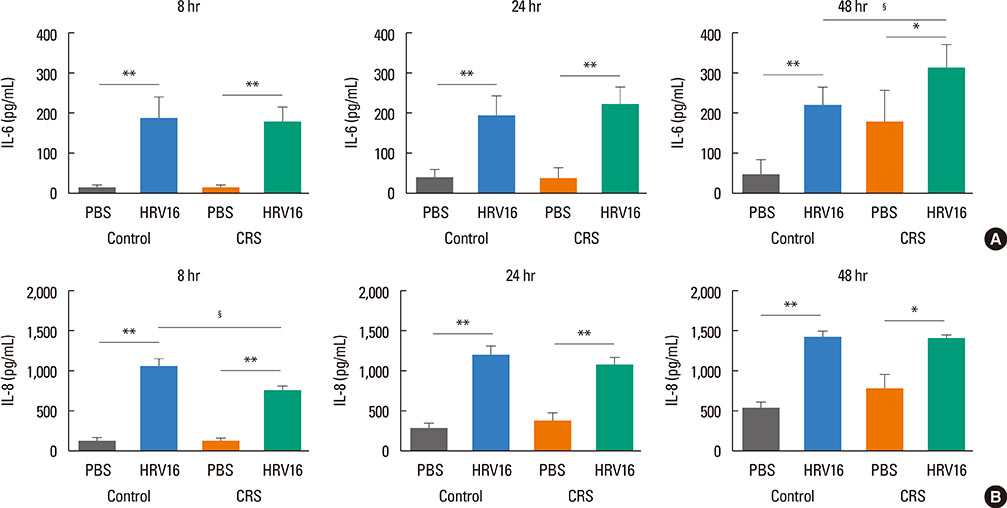
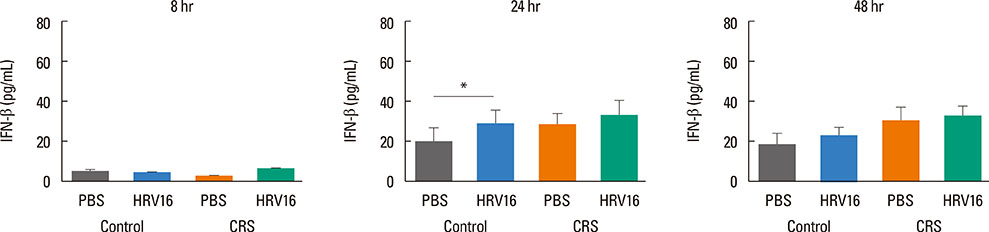
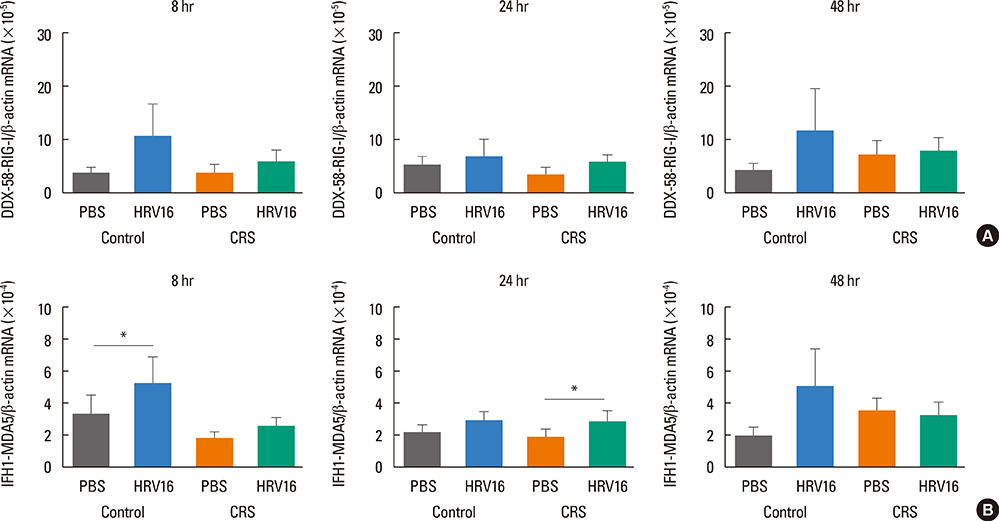
HNECs, isolated from turbinate tissue of 13 patients with CRS and 14 non-CRS controls, were infected with HRV16 for 4 hours. The HRV titer, LDH activity, production of proinflammatory cytokines and IFN-beta proteins, and expression levels of RIG-I and MDA5 mRNA were assessed at 8, 24, and 48 hours after HRV16 infection.
RESULTS
The reduction in viral titer was slightly delayed in the CRS group compared to the non-CRS control group. IL-6 and IL-8 were significantly increased to a similar extent in both groups after HRV infection. In the control group, IFN-beta production and MDA5 mRNA expression were significantly increased at 8 and 24 hours after HRV16 infection, respectively. By contrast, in the CRS group, IFN-beta was not induced by HRV infection; however, HRV-induced MDA5 mRNA expression was increased, but the increase was slightly delayed compared to the non-CRS control group. The RIG-I mRNA level was not significantly increased by HRV16 infection in either group.
CONCLUSIONS
HRV-induced secretion of proinflammatory cytokines in CRS patients was not different from that in the non-CRS controls. However, reductions in viral titer, IFN-beta secretion, and MDA5 mRNA expression in response to HRV infection in CRS patients were slightly impaired compared to those in the controls, suggesting that HRV clearance in CRS patients might be slightly deficient.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Viral Infections and Associated Factors That Promote Acute Exacerbations of Asthma
Chang-Keun Kim, Zak Callaway, James E. Gern
Allergy Asthma Immunol Res. 2018;10(1):12-17. doi: 10.4168/aair.2018.10.1.12.The Role of Viruses in the Inception of Chronic Rhinosinusitis
Hyeon Seung Lee, Sophia J Volpe, Eugene H Chang
Clin Exp Otorhinolaryngol. 2022;15(4):310-318. doi: 10.21053/ceo.2022.01004.
Reference
-
1. Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011; 24:210–229.2. Winther B, Gwaltney JM Jr, Mygind N, Hendley JO. Viral-induced rhinitis. Am J Rhinol. 1998; 12:17–20.3. Greenberg SB. Respiratory viral infections in adults. Curr Opin Pulm Med. 2002; 8:201–208.4. Greenberg SB. Respiratory consequences of rhinovirus infection. Arch Intern Med. 2003; 163:278–284.5. Triantafilou K, Vakakis E, Richer EA, Evans GL, Villiers JP, Triantafilou M. Human rhinovirus recognition in non-immune cells is mediated by Toll-like receptors and MDA-5, which trigger a synergetic pro-inflammatory immune response. Virulence. 2011; 2:22–29.6. Slater L, Bartlett NW, Haas JJ, Zhu J, Message SD, Walton RP, et al. Coordinated role of TLR3, RIG-I and MDA5 in the innate response to rhinovirus in bronchial epithelium. PLoS Pathog. 2010; 6:e1001178.7. Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006; 441:101–105.8. Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003; 424:516–523.9. Message SD, Johnston SL. Host defense function of the airway epithelium in health and disease: clinical background. J Leukoc Biol. 2004; 75:5–17.10. Yeo NK, Hwang YJ, Kim ST, Kwon HJ, Jang YJ. Asian sand dust enhances rhinovirus-induced cytokine secretion and viral replication in human nasal epithelial cells. Inhal Toxicol. 2010; 22:1038–1045.11. Rosenfeld RM, Andes D, Bhattacharyya N, Cheung D, Eisenberg S, Ganiats TG, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007; 137:S1–S31.12. Jang YJ, Kwon HJ, Park HW, Lee BJ. Detection of rhinovirus in turbinate epithelial cells of chronic sinusitis. Am J Rhinol. 2006; 20:634–636.13. Cho GS, Moon BJ, Lee BJ, Gong CH, Kim NH, Kim YS, et al. High rates of detection of respiratory viruses in the nasal washes and mucosae of patients with chronic rhinosinusitis. J Clin Microbiol. 2013; 51:979–984.14. Hellings PW, Hens G. Rhinosinusitis and the lower airways. Immunol Allergy Clin North Am. 2009; 29:733–740.15. Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004; 114:239–247.16. Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol. 2012; 129:1499–1505.e5.17. Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005; 201:937–947.18. Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo MB, et al. Rhinovirus 16-induced IFN-α and IFN-β are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012; 129:1506–1514.e6.19. Jang YJ, Wang JH, Kim JS, Kwon HJ, Yeo NK, Lee BJ. Levocetirizine inhibits rhinovirus-induced ICAM-1 and cytokine expression and viral replication in airway epithelial cells. Antiviral Res. 2009; 81:226–233.20. Wang JH, Kwon HJ, Chung YS, Lee BJ, Jang YJ. Infection rate and virus-induced cytokine secretion in experimental rhinovirus infection in mucosal organ culture: comparison between specimens from patients with chronic rhinosinusitis with nasal polyps and those from normal subjects. Arch Otolaryngol Head Neck Surg. 2008; 134:424–427.21. Kim JH, Kwon HJ, Jang YJ. Effects of rhinovirus infection on the expression and function of cystic fibrosis transmembrane conductance regulator and epithelial sodium channel in human nasal mucosa. Ann Allergy Asthma Immunol. 2012; 108:182–187.22. Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004; 114:155–212.23. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50:1–12.24. Lopez-Souza N, Favoreto S, Wong H, Ward T, Yagi S, Schnurr D, et al. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J Allergy Clin Immunol. 2009; 123:1384–1390.e2.25. Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009; 183:6989–6997.26. Proud D, Hudy MH, Wiehler S, Zaheer RS, Amin MA, Pelikan JB, et al. Cigarette smoke modulates expression of human rhinovirus-induced airway epithelial host defense genes. PLoS One. 2012; 7:e40762.27. Bogefors J, Kvarnhammar AM, Latif L, Petterson T, Uddman R, Cardell LO. Retinoic acid-inducible gene 1-like receptors in the upper respiratory tract. Am J Rhinol Allergy. 2011; 25:e262–e267.28. Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008; 20:17–22.29. Edwards MR, Regamey N, Vareille M, Kieninger E, Gupta A, Shoemark A, et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 2013; 6:797–806.30. Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011; 183:734–742.31. Vareille M, Kieninger E, Alves MP, Kopf BS, Möller A, Geiser T, et al. Impaired type I and type III interferon induction and rhinovirus control in human cystic fibrosis airway epithelial cells. Thorax. 2012; 67:517–525.32. Gaajetaan GR, Geelen TH, Vernooy JH, Dentener MA, Reynaert NL, Rohde GG, et al. Interferon-β induces a long-lasting antiviral state in human respiratory epithelial cells. J Infect. 2013; 66:163–169.33. Okabayashi T, Kojima T, Masaki T, Yokota S, Imaizumi T, Tsutsumi H, et al. Type-III interferon, not type-I, is the predominant interferon induced by respiratory viruses in nasal epithelial cells. Virus Res. 2011; 160:360–366.34. Scagnolari C, Midulla F, Pierangeli A, Moretti C, Bonci E, Berardi R, et al. Gene expression of nucleic acid-sensing pattern recognition receptors in children hospitalized for respiratory syncytial virus-associated acute bronchiolitis. Clin Vaccine Immunol. 2009; 16:816–823.35. Luo B, Feng L, Jintao D, Yafeng L, Shixi L, Nan Z, et al. Immunopathology features of chronic rhinosinusitis in high-altitude dwelling Tibetans. Allergy Rhinol (Providence). 2013; 4:e69–e76.36. Shi LL, Xiong P, Zhang L, Cao PP, Liao B, Lu X, et al. Features of airway remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy. 2013; 68:101–109.37. Yeo NK, Jang YJ. Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells. Laryngoscope. 2010; 120:346–352.38. Jang YJ, Lee YH, Shin SH. Rhinovirus-infected nasal polyp epithelial cells: effect on the activation and migration of eosinophils by airborne fungi. Ann Allergy Asthma Immunol. 2010; 104:434–439.39. Min JY, Shin SH, Kwon HJ, Jang YJ. Levocetirizine inhibits rhinovirus-induced bacterial adhesion to nasal epithelial cells through down-regulation of cell adhesion molecules. Ann Allergy Asthma Immunol. 2012; 108:44–48.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increase of Rhinovirus Replication in Airway Epithelial Cells by Staphylococcal Enterotoxin A and B
- Comparison of Rhinovirus Infection Rate and Virus-induced Cytokine Secretion between Nasal Polyp Mucosae and Normal Sphenoid Sinus Mucosae Organ Culture Model
- Effects of Fungi and Eosinophils on Mucin Gene Expression in Rhinovirus-Infected Nasal Epithelial Cells
- The role of cytokines in rhinosinusitis
- Tissue Remodeling in Rhinosinusitis