Allergy Asthma Immunol Res.
2014 Mar;6(2):149-155. 10.4168/aair.2014.6.2.149.
Effects of Fungi and Eosinophils on Mucin Gene Expression in Rhinovirus-Infected Nasal Epithelial Cells
- Affiliations
-
- 1Department of Otolaryngology, Catholic University of Daegu, School of Medicine, Daegu, Korea. hsseung@cu.ac.kr
- KMID: 2260260
- DOI: http://doi.org/10.4168/aair.2014.6.2.149
Abstract
- PURPOSE
Fungi, rhinoviruses (RVs), and eosinophils are associated with upper respiratory diseases. We evaluated the effects of fungal stimulation and eosinophil co-culture on the expression of mucin genes in RV-infected nasal polyp epithelial cells.
METHODS
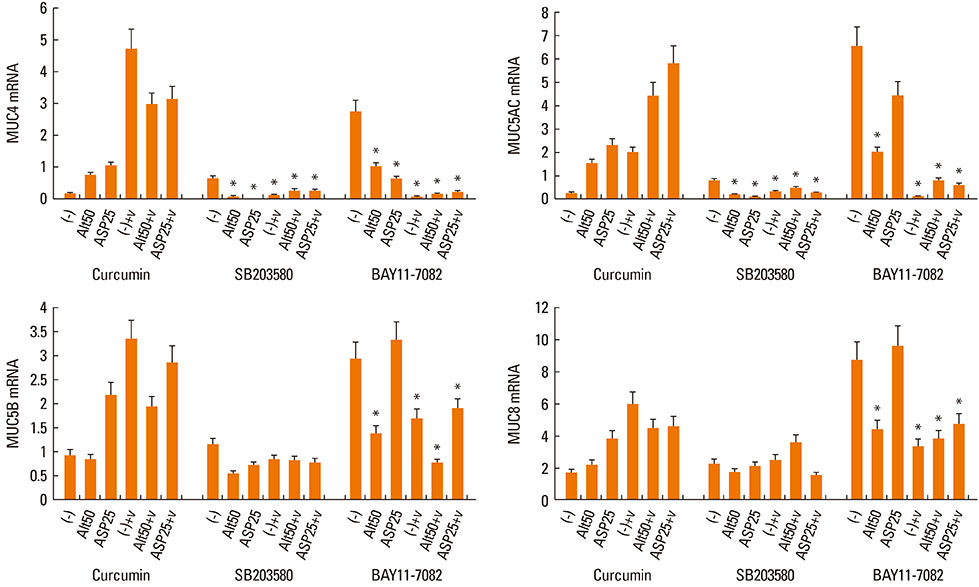
Nasal polyp epithelial cells were obtained from chronic rhinosinusitis patients. Cultured epithelial cells were stimulated with Alternaria and Aspergillus with or without RV-16 infection. The epithelial cells were co-cultured with eosinophils for 16 h. MUC4, MUC5AC, MUC5B, and MUC8 mRNA expressions in the epithelial cells were quantified using real-time RT-PCR. To determine the underlying mechanism, nuclear factor-kappaB (NF-kappaB), activator protein-1 (AP-1), and mitogen-activated protein kinase (MAPK) inhibitors were used to inhibit mucin gene expression.
RESULTS
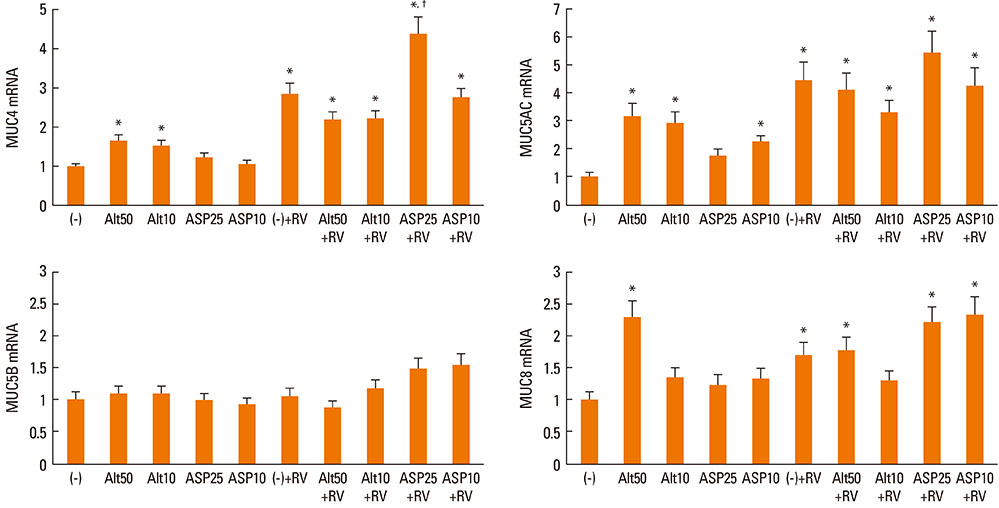
Fungi and RV-16 induced mucin gene expression in nasal polyp epithelial cells. However, there was no synergistic increase in mucin gene expression, with the exception of MUC4 mRNA expression stimulated by 25 microg/mL Aspergillus. When RV-16-infected epithelial cells were stimulated with fungi and then co-cultured with eosinophils, MUC4, MUC5B, and MUC8 mRNA expressions increased. Mucin gene expression was inhibited by NF-kappaB inhibitors.
CONCLUSIONS
RV-16, airborne fungi, and eosinophils may exacerbate the inflammatory process in nasal mucosal diseases by enhancing mucin gene expression.
MeSH Terms
Figure
Reference
-
1. Andersson M, Downs S, Mitakakis T, Leuppi J, Marks G. Natural exposure to Alternaria spores induces allergic rhinitis symptoms in sensitized children. Pediatr Allergy Immunol. 2003; 14:100–105.2. Shin SH, Lee YH, Jeon CH. Protease-dependent activation of nasal polyp epithelial cells by airborne fungi leads to migration of eosinophils and neutrophils. Acta Otolaryngol. 2006; 126:1286–1294.3. Igarashi Y, Skoner DP, Doyle WJ, White MV, Fireman P, Kaliner MA. Analysis of nasal secretions during experimental rhinovirus upper respiratory infections. J Allergy Clin Immunol. 1993; 92:722–731.4. Johnston SL, Papi A, Bates PJ, Mastronarde JG, Monick MM, Hunninghake GW. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J Immunol. 1998; 160:6172–6181.5. Jang YJ, Kwon HJ, Park HW, Lee BJ. Detection of rhinovirus in turbinate epithelial cells of chronic sinusitis. Am J Rhinol. 2006; 20:634–636.6. Stoop AE, van der Heijden HA, Biewenga J, van der Baan S. Eosinophils in nasal polyps and nasal mucosa: an immunohistochemical study. J Allergy Clin Immunol. 1993; 91:616–622.7. Khan DA, Cody DT 2nd, George TJ, Gleich GJ, Leiferman KM. Allergic fungal sinusitis: an immunohistologic analysis. J Allergy Clin Immunol. 2000; 106:1096–1101.8. Ali MS, Wilson JA, Bennett M, Pearson JP. Mucin gene expression in nasal polyps. Acta Otolaryngol. 2005; 125:618–624.9. Kim JY, Kim CH, Kim KS, Choi YS, Lee JG, Yoon JH. Extracellular signal-regulated kinase is involved in tumor necrosis factor-alpha-induced MUC5AC gene expression in cultured human nasal polyp epithelial cells. Acta Otolaryngol. 2004; 124:953–957.10. Jang YJ, Wang JH, Kim JS, Kwon HJ, Yeo NK, Lee BJ. Levocetirizine inhibits rhinovirus-induced ICAM-1 and cytokine expression and viral replication in airway epithelial cells. Antiviral Res. 2009; 81:226–233.11. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.12. Martínez-Antón A, Debolós C, Garrido M, Roca-Ferrer J, Barranco C, Alobid I, Xaubet A, Picado C, Mullol J. Mucin genes have different expression patterns in healthy and diseased upper airway mucosa. Clin Exp Allergy. 2006; 36:448–457.13. Ding GQ, Zheng CQ. The expression of MUC5AC and MUC5B mucin genes in the mucosa of chronic rhinosinusitis and nasal polyposis. Am J Rhinol. 2007; 21:359–366.14. Xaubet A, Mullol J, López E, Roca-Ferrer J, Rozman M, Carrión T, Fabra JM, Picado C. Comparison of the role of nasal polyp and normal nasal mucosal epithelial cells on in vitro eosinophil survival. Mediation by GM-CSF and inhibition by dexamethasone. Clin Exp Allergy. 1994; 24:307–317.15. Mullol J, Xaubet A, Gaya A, Roca-Ferrer J, López E, Fernàndez JC, Fernàndez MD, Picado C. Cytokine gene expression and release from epithelial cells. A comparison study between healthy nasal mucosa and nasal polyps. Clin Exp Allergy. 1995; 25:607–615.16. Kim SS, Kim KS, Lee JG, Park IY, Koo JS, Yoon JH. Levels of intracellular protein and messenger RNA of mucin and lysozyme in normal human nasal and polyp epithelium. Laryngoscope. 2000; 110:276–280.17. Jang YJ, Lee YH, Shin SH. Rhinovirus-infected nasal polyp epithelial cells: effect on the activation and migration of eosinophils by airborne fungi. Ann Allergy Asthma Immunol. 2010; 104:434–439.18. Shin SH, Lee YH. Airborne fungi induce nasal polyp epithelial cell activation and toll-like receptor expression. Int Arch Allergy Immunol. 2010; 153:46–52.19. Basbaum C, Lemjabbar H, Longphre M, Li D, Gensch E, McNamara N. Control of mucin transcription by diverse injury-induced signaling pathways. Am J Respir Crit Care Med. 1999; 160:S44–S48.20. Shin SH, Lee SH, Jeong HS, Kita H. The effect of nasal polyp epithelial cells on eosinophil activation. Laryngoscope. 2003; 113:1374–1377.21. Takafuji S, Ohtoshi T, Takizawa H, Tadokoro K, Ito K. Eosinophil degranulation in the presence of bronchial epithelial cells. Effect of cytokines and role of adhesion. J Immunol. 1996; 156:3980–3985.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rhinovirus-Induced Mucin Gene Expression in Airway Epithelial Cells
- Roxithromycin Suppresses MUC5B/8 Mucin Genes and Mucin Production in Airway Epithelial Cells
- Effect of Clarithromycin on Rhinovirus-16 Infection in A549 Cells
- Comparison of Mucin and Lysozyme Expression between Human in vivo Nasal Epithelial Cells and Cultured Nasal Epithelial Cells
- The Effects of Eosinophils Activated with Airborne Fungi on Nasal Epithelial Cells