Korean Circ J.
2014 May;44(3):177-183. 10.4070/kcj.2014.44.3.177.
Angiopoietin-Like 4 Is Involved in the Poor Angiogenic Potential of High Glucose-Insulted Bone Marrow Stem Cells
- Affiliations
-
- 1Research Laboratory of Cardiovascular Regeneration, Chonnam National University Hospital, Gwangju, Korea. cecilyk@hanmail.net
- 2Cardiovascular Convergence Research Center, Chonnam National University Hospital, Gwangju, Korea.
- 3Center for Molecular Medicine, Graduate School, Chonnam National University, Gwangju, Korea.
- 4Department of Pharmacology and Medical Research Center for Gene Regulation, Chonnam National University Medical School, Gwangju, Korea.
- 5Department of Cardiology, Chonnam National University Hospital, Gwangju, Korea.
- KMID: 2145479
- DOI: http://doi.org/10.4070/kcj.2014.44.3.177
Abstract
- BACKGROUND AND OBJECTIVES
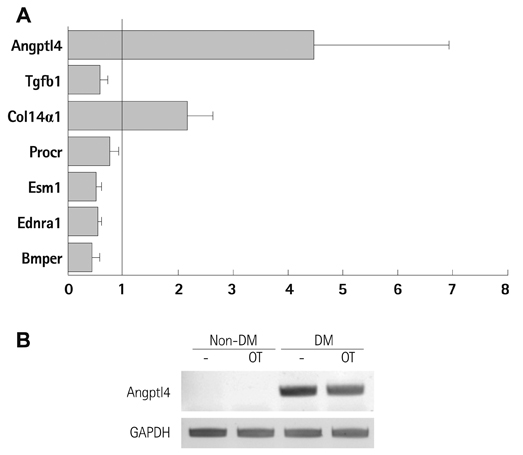
Diabetes is reported to reduce the function or number of progenitor cells. We compared the gene expression patterns of bone marrow-derived mesenchymal stem cells from diabetic (DM-BMCs) and healthy (non-DM-BMCs) rats and suggested Angiopoietin-like 4 (Angptl4) could be a responsible factor for impaired angiogenesis of DM-BMCs.
SUBJECTS AND METHODS
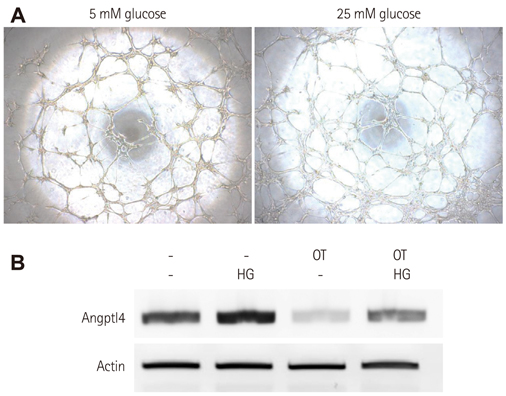
BMCs were isolated from DM or non-DM rat, and in vitro angiogenesis activity was compared by tube formation assay on Matrigel and complementary deoxyribonucleic acid expression was analyzed by microarray with or without oxytocin treatment. Human BMCs (hBMCs) were treated with high glucose, and were performed polymerase chain reaction, Western blot, and enzyme-linked immunosorbent assay. Angptl4 plasmid DNA and micro ribonucleic acid-132 (miR-132) were transfected to immortalized hBMCs.
RESULTS
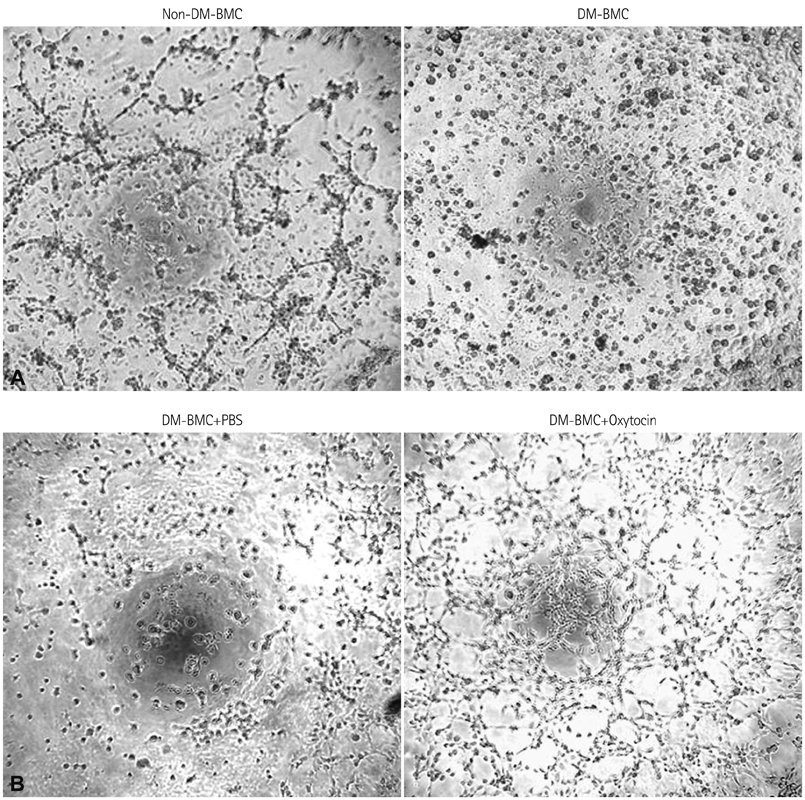
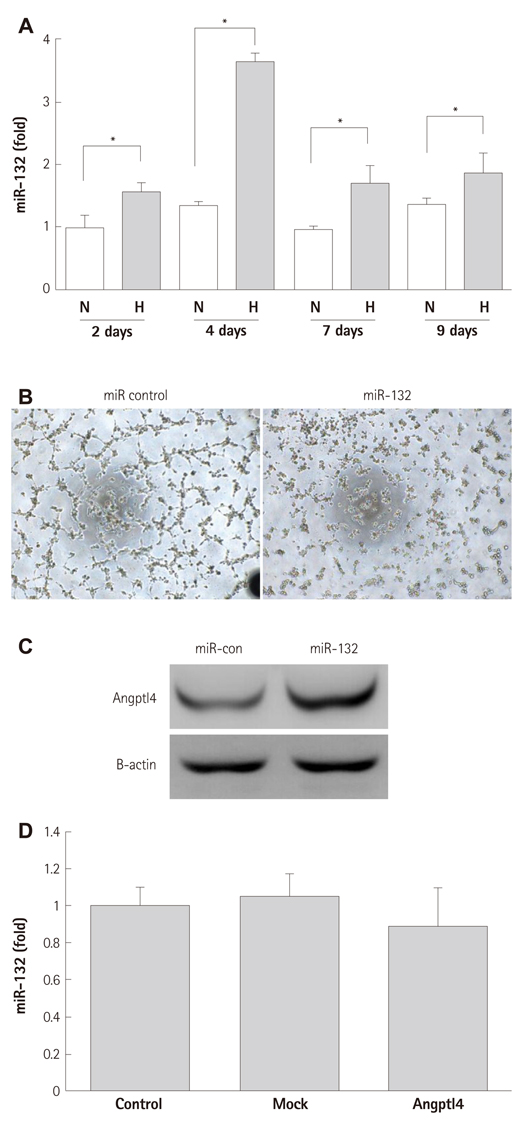
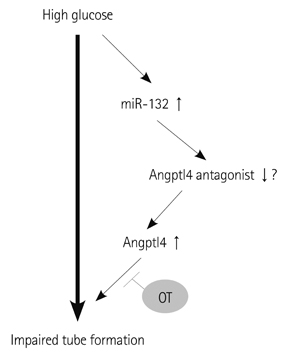
In vitro angiogenesis assay showed the impaired tube formation in DM-BMCs, and slightly recovery by oxytocin treatment. Angptl4, an adipokine, was upregulated in DM-BMCs compared to non-DM-BMCs. Oxytocin treatment reduced Angptl4 in DM-BMCs. In hBMCs, overexpression of Angptl4 attenuated the tube formation. In addition to Angptl4, miR-132 was increased by high glucose treatment. Collectively, high glucose resulted in impaired tube formation through miR-132 induction and Angptl4 upregulation in BMCs.
CONCLUSION
Our results show that the angiogenic activity of BMCs is impaired by high glucose stress, which would be mediated by Angptl4 and miR-132.
Keyword
MeSH Terms
Figure
Reference
-
1. Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006; 12:459–465.2. Kim YS, Kwon JS, Hong MH, et al. Restoration of angiogenic capacity of diabetes-insulted mesenchymal stem cells by oxytocin. BMC Cell Biol. 2013; 14:38.3. Fadini GP, Sartore S, Schiavon M, et al. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006; 49:3075–3084.4. Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003; 348:593–600.5. Porrello ER, Olson EN. Building a new heart from old parts: stem cell turnover in the aging heart. Circ Res. 2010; 107:1292–1294.6. Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999; 100:1134–1146.7. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011; 123:e18–e209.8. Orchard TJ, Costacou T, Kretowski A, Nesto RW. Type 1 diabetes and coronary artery disease. Diabetes Care. 2006; 29:2528–2538.9. Cull CA, Jensen CC, Retnakaran R, Holman RR. Impact of the metabolic syndrome on macrovascular and microvascular outcomes in type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study 78. Circulation. 2007; 116:2119–2126.10. Rivard A, Silver M, Chen D, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999; 154:355–363.11. Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004; 53:195–199.12. Dernbach E, Randriamboavonjy V, Fleming I, Zeiher AM, Dimmeler S, Urbich C. Impaired interaction of platelets with endothelial progenitor cells in patients with cardiovascular risk factors. Basic Res Cardiol. 2008; 103:572–581.13. Yang YH, Wang Y, Lam KS, et al. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008; 28:835–840.14. Ito Y, Oike Y, Yasunaga K, et al. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 2003; 63:6651–6657.15. Ma T, Jham BC, Hu J, et al. Viral G protein-coupled receptor up-regulates Angiopoietin-like 4 promoting angiogenesis and vascular permeability in Kaposi's sarcoma. Proc Natl Acad Sci U S A. 2010; 107:14363–14368.16. Okochi-Takada E, Hattori N, Tsukamoto T, et al. ANGPTL4 is a secreted tumor suppressor that inhibits angiogenesis. Oncogene. 2013; [Epub ahead of print].17. Anand S, Majeti BK, Acevedo LM, et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010; 16:909–914.18. Westenskow PD, Kurihara T, Aguilar E, et al. Ras pathway inhibition prevents neovascularization by repressing endothelial cell sprouting. J Clin Invest. 2013; 123:4900–4908.19. Devalliere J, Chang WG, Andrejecsk JW, et al. Sustained delivery of proangiogenic microRNA-132 by nanoparticle transfection improves endothelial cell transplantation. FASEB J. 2014; 28:908–922.20. Choe N, Kwon JS, Kim JR, et al. The microRNA miR-132 targets Lrrfip1 to block vascular smooth muscle cell proliferation and neointimal hyperplasia. Atherosclerosis. 2013; 229:348–355.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Angiogenic Stem Cells in Bone Regeneration
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Cell Therapy for Diabetic Neuropathy Using Adult Stem or Progenitor Cells
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Osteogenic and Angiogenic Potency of VEGF165-Transfected Canine Bone Marrow Mesenchymal Cells Combined with Coral Hydroxyapatite in Vitro