J Korean Med Sci.
2015 Mar;30(3):308-316. 10.3346/jkms.2015.30.3.308.
P70S6K and Elf4E Dual Inhibition Is Essential to Control Bladder Tumor Growth and Progression in Orthotopic Mouse Non-muscle Invasive Bladder Tumor Model
- Affiliations
-
- 1Department of Urology, Chung-Ang University College of Medicine, Seoul, Korea. caucih@cau.ac.kr
- 2Biomedical Science, Department of Medicine, Chung-Ang University Graduate School, Seoul, Korea.
- 3Center for Prostate Cancer, Research Institute, National Cancer Center, Goyang, Korea.
- 4Genitourinary Cancer Branch, Research Institute, National Cancer Center, Goyang, Korea.
- 5Department of Pathology, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2133310
- DOI: http://doi.org/10.3346/jkms.2015.30.3.308
Abstract
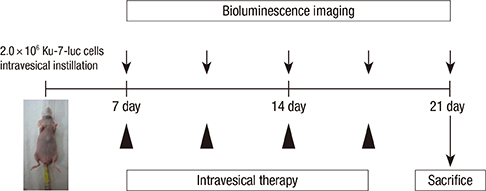
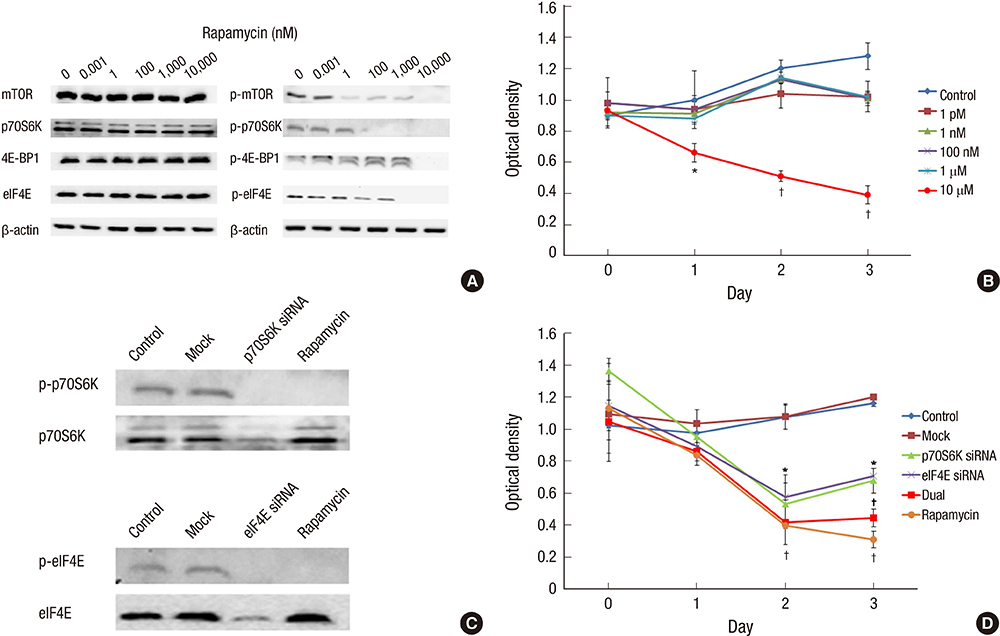
- We investigated how the dual inhibition of the molecular mechanism of the mammalian target of the rapamycin (mTOR) downstreams, P70S6 kinase (P70S6K) and eukaryotic initiation factor 4E (eIF4E), can lead to a suppression of the proliferation and progression of urothelial carcinoma (UC) in an orthotopic mouse non-muscle invasive bladder tumor (NMIBT) model. A KU-7-luc cell intravesically instilled orthotopic mouse NMIBC model was monitored using bioluminescence imaging (BLI) in vivo by interfering with different molecular components using rapamycin and siRNA technology. We then analyzed the effects on molecular activation status, cell growth, proliferation, and progression. A high concentration of rapamycin (10 microM) blocked both P70S6K and elF4E phosphorylation and inhibited cell proliferation in the KU-7-luc cells. It also reduced cell viability and proliferation more than the transfection of siRNA against p70S6K or elF4E. The groups with dual p70S6K and elF4E siRNA, and rapamycin reduced tumor volume and lamina propria invasion more than the groups with p70S6K or elF4E siRNA instillation, although all groups reduced photon density compared to the control. These findings suggest that both the mTOR pathway downstream of eIF4E and p70S6K can be successfully inhibited by high dose rapamycin only, and p70S6K and Elf4E dual inhibition is essential to control bladder tumor growth and progression.
MeSH Terms
-
Animals
Cell Line
Cell Proliferation/drug effects/genetics
Cell Survival/drug effects
Disease Progression
Eukaryotic Initiation Factor-4E/*antagonists & inhibitors/genetics
Female
Mice
Mice, Nude
Mucous Membrane/pathology
Phosphorylation/drug effects
RNA Interference
RNA, Small Interfering
Ribosomal Protein S6 Kinases, 70-kDa/*antagonists & inhibitors/genetics
Signal Transduction/drug effects
Sirolimus/*pharmacology
TOR Serine-Threonine Kinases/*antagonists & inhibitors/metabolism
Urinary Bladder Neoplasms/genetics/*pathology
Urothelium/pathology
Eukaryotic Initiation Factor-4E
RNA, Small Interfering
TOR Serine-Threonine Kinases
Ribosomal Protein S6 Kinases, 70-kDa
Sirolimus
Figure
Reference
-
1. Sharir S. Update on clinical and radiological staging and surveillance of bladder cancer. Can J Urol. 2006; 13:71–76.2. Bulbul MA, Husseini N, Houjaij A. Superficial bladder cancer epidemiology, diagnosis and management. J Med Liban. 2005; 53:107–113.3. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59:225–249.4. Murta-Nascimento C, Schmitz-Dräger BJ, Zeegers MP, Steineck G, Kogevinas M, Real FX, Malats N. Epidemiology of urinary bladder cancer: from tumor development to patient's death. World J Urol. 2007; 25:285–295.5. Catalona WJ, Ratliff TL. Bacillus Calmette-Guerin and superficial bladder cancer. Clinical experience and mechanism of action. Surg Annu. 1990; 22:363–378.6. Holmäng S, Hedelin H, Anderström C, Holmberg E, Busch C, Johansson SL. Recurrence and progression in low grade papillary urothelial tumors. J Urol. 1999; 162:702–707.7. Cookson MS, Herr HW, Zhang ZF, Soloway S, Sogani PC, Fair WR. The treated natural history of high risk superficial bladder cancer: 15-year outcome. J Urol. 1997; 158:62–67.8. Kavoussi LR, Torrence RJ, Gillen DP, Hudson MA, Haaff EO, Dresner SM, Ratliff TL, Catalona WJ. Results of 6 weekly intravesical bacillus Calmette-Guerin instillations on the treatment of superficial bladder tumors. J Urol. 1988; 139:935–940.9. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999; 13:2905–2927.10. Ching CB, Hansel DE. Expanding therapeutic targets in bladder cancer: the PI3K/Akt/mTOR pathway. Lab Invest. 2010; 90:1406–1414.11. Rousseau D, Gingras AC, Pause A, Sonenberg N. The eIF4E-binding proteins 1 and 2 are negative regulators of cell growth. Oncogene. 1996; 13:2415–2420.12. Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5'TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997; 16:3693–3704.13. Park SJ, Lee TJ, Chang IH. Role of the mTOR pathway in the progression and recurrence of bladder cancer: an immunohistochemical tissue microarray study. Korean J Urol. 2011; 52:466–473.14. Kwon JK, Kim SJ, Kim JH, Lee KM, Chang IH. Dual inhibition by S6K1 and Elf4E is essential for controlling cellular growth and invasion in bladder cancer. Urol Oncol. 2014; 32:51.e27–51.e35.15. Yang XH, Ren LS, Wang GP, Zhao LL, Zhang H, Mi ZG, Bai X. A new method of establishing orthotopic bladder transplantable tumor in mice. Cancer Biol Med. 2012; 9:261–265.16. Hadaschik BA, Black PC, Sea JC, Metwalli AR, Fazli L, Dinney CP, Gleave ME, So AI. A validated mouse model for orthotopic bladder cancer using transurethral tumour inoculation and bioluminescence imaging. BJU Int. 2007; 100:1377–1384.17. Kim SJ, Seo HK, Seo HH, Lee SJ, Kwon JK, Lee TJ, Chi BH, Chang IH. Establishment of an orthotopic mouse non-muscle invasive bladder cancer model expressing the mammalian target of rapamycin signaling pathway. J Korean Med Sci. 2014; 29:343–350.18. Hadaschik BA, Zhang K, So AI, Fazli L, Jia W, Bell JC, Gleave ME, Rennie PS. Oncolytic vesicular stomatitis viruses are potent agents for intravesical treatment of high-risk bladder cancer. Cancer Res. 2008; 68:4506–4510.19. Brunn GJ, Fadden P, Haystead TA, Lawrence JC Jr. The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J Biol Chem. 1997; 272:32547–32550.20. Tang S, Hu RG, Liu WY, Ruan KC. Non-specific depurination activity of saporin-S6, a ribosome-inactivating protein, under acidic conditions. Biol Chem. 2000; 381:769–772.21. Seufferlein T, Rozengurt E. Rapamycin inhibits constitutive p70s6k phosphorylation, cell proliferation, and colony formation in small cell lung cancer cells. Cancer Res. 1996; 56:3895–3897.22. Grewe M, Gansauge F, Schmid RM, Adler G, Seufferlein T. Regulation of cell growth and cyclin D1 expression by the constitutively active FRAP-p70s6K pathway in human pancreatic cancer cells. Cancer Res. 1999; 59:3581–3587.23. Mansure JJ, Nassim R, Chevalier S, Rocha J, Scarlata E, Kassouf W. Inhibition of mammalian target of rapamycin as a therapeutic strategy in the management of bladder cancer. Cancer Biol Ther. 2009; 8:2339–2347.24. Jäger W, Horiguchi Y, Shah J, Hayashi T, Awrey S, Gust KM, Hadaschik BA, Matsui Y, Anderson S, Bell RH, et al. Hiding in plain view: genetic profiling reveals decades old cross contamination of bladder cancer cell line KU7 with HeLa. J Urol. 2013; 190:1404–1409.25. Kang MR, Yang G, Charisse K, Epstein-Barash H, Manoharan M, Li LC. An orthotopic bladder tumor model and the evaluation of intravesical saRNA treatment. J Vis Exp. 2012.26. Watanabe T, Shinohara N, Sazawa A, Harabayashi T, Ogiso Y, Koyanagi T, Takiguchi M, Hashimoto A, Kuzumaki N, Yamashita M, et al. An improved intravesical model using human bladder cancer cell lines to optimize gene and other therapies. Cancer Gene Ther. 2000; 7:1575–1580.27. Jung SY, Willard ST. Quantitative bioluminescence imaging of transgene expression in intact porcine antral follicles in vitro. Reprod Biol Endocrinol. 2014; 12:11.28. Luan FL, Hojo M, Maluccio M, Yamaji K, Suthanthiran M. Rapamycin blocks tumor progression: unlinking immunosuppression from antitumor efficacy. Transplantation. 2002; 73:1565–1572.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tumor Establishment Features of Orthotopic Murine Bladder Cancer Models
- Accuracy of Vesical Imaging-Reporting and Data System for muscle-invasive bladder cancer detection from multiparametric magnetic resonance imaging
- The Factors Influencing Prognosis of Superficial Bladder Tumor
- Predictive Value of Urinary Cytology in the Recurrence and the Progression of Superficial Bladder Cancer
- Autophagy and urothelial carcinoma of the bladder: A review