Korean J Urol.
2012 Jun;53(6):396-400.
Tumor Establishment Features of Orthotopic Murine Bladder Cancer Models
- Affiliations
-
- 1Department of Urology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. urojoo@dreamwiz.com
Abstract
- PURPOSE
Animal tumor models are important for the evaluation of novel therapeutic modalities. Since the initial report of an orthotopic bladder tumor model, several modifications have been proposed to improve the tumor take rate. Here we compared the HCl-pretreated and electrocauterization-pretreated orthotopic murine bladder tumor models.
MATERIALS AND METHODS
MBT-2 murine bladder cancer cells were transurethrally implanted in the bladder of syngeneic C3H/He mice. The mice were divided into three groups according to pretreatment methods (electrocautery, HCl, and control group) and were subjected to pretreatment before instillation of MBT-2 tumor cells into the bladder. Mice were sacrificed on day 21, and bladders were harvested, weighed, and examined histopathologically.
RESULTS
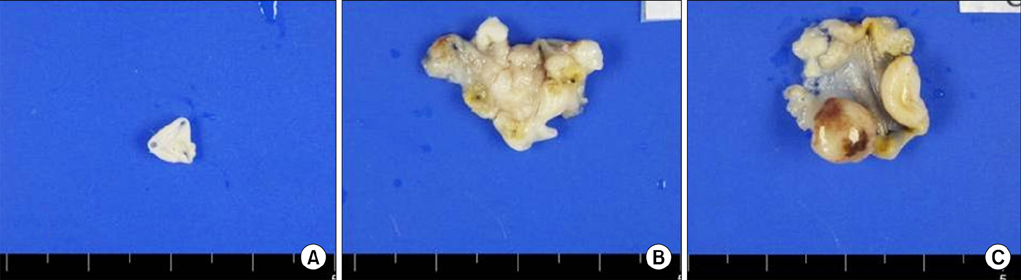
The tumor take rate of the control, electrocautery, and HCl groups was 0%, 54%, and 100%, respectively. The tumor take rate of the HCl group was significantly higher than that of the control group (p<0.01) and the electrocautery group (p=0.01). Pathologic reports revealed that all established bladder tumors were high-grade papillary urothelial carcinomas.
CONCLUSIONS
The HCl pretreatment model was a preferable murine bladder tumor model for evaluating further therapeutic interventions.
MeSH Terms
Figure
Reference
-
1. Ratliff TL. Role of animal models in understanding intravesical therapy with bacille Calmette-Guerin. Clin Infect Dis. 2000. 31:Suppl 3. S106–S108.2. McCue PA, Gomella LG, Veltri RW, Marley GM, Miller MC, Lattime EC. Development of secondary structure, growth characteristics and cytogenetic analysis of human transitional cell carcinoma xenografts in scid/scid mice. J Urol. 1996. 155:1128–1132.3. Xiao Z, McCallum TJ, Brown KM, Miller GG, Halls SB, Parney I, et al. Characterization of a novel transplantable orthotopic rat bladder transitional cell tumour model. Br J Cancer. 1999. 81:638–646.4. Chan ES, Patel AR, Smith AK, Klein JB, Thomas AA, Heston WD, et al. Optimizing orthotopic bladder tumor implantation in a syngeneic mouse model. J Urol. 2009. 182:2926–2931.5. Soloway MS. Intravesical and systemic chemotherapy of murine bladder cancer. Cancer Res. 1977. 37(8 Pt 2):2918–2929.6. Shapiro A, Kelley DR, Oakley DM, Catalona WJ, Ratliff TL. Technical factors affecting the reproducibility of intravesical mouse bladder tumor implantation during therapy with Bacillus Calmette-Guerin. Cancer Res. 1984. 44:3051–3054.7. Horinaga M, Harsch KM, Fukuyama R, Heston W, Larchian W. Intravesical interleukin-12 gene therapy in an orthotopic bladder cancer model. Urology. 2005. 66:461–466.8. Janik P, Briand P, Hartmann NR. The effect of estrone-progesterone treatment on cell proliferation kinetics of hormone-dependent GR mouse mammary tumors. Cancer Res. 1975. 35:3698–3704.9. Soloway MS. Single and combination chemotherapy for primary murine bladder cancer. Cancer. 1975. 36:333–340.10. Williams PD, Murphy GP. Experimental bladder tumor induction, propagation, and therapy. Urology. 1976. 8:39–42.11. Park HS, Cheon J, Yoon DK, Koh SK, Kim HK. The changes of histopathology, proliferation activity, and nuclear DNA content N-butyl-N-(4-hydroxybuty1) nitrosamine (BBN) induced bladder cancer in rats. Korean J Urol. 1995. 36:683–691.12. Jin JH, Lee GH, Kim HJ. Chemopreventive effect of aspirin on N-Butyl-N-(4-hydroxybutyl) nitrosamine induced preneoplastic lesions in rat bladder. Korean J Urol. 2001. 42:631–635.13. Hubbell HR, Kvalnes-Krick K, Carter WA, Strayer DR. Antiproliferative and immunomodulatory actions of beta-interferon and double-stranded RNA, individually and in combination, on human bladder tumor xenografts in nude mice. Cancer Res. 1985. 45:2481–2486.14. Huland H, Otto U, von Paleske A. Chemotherapy and human bladder carcinoma transplanted into NMRI nu/nu mice. J Urol. 1985. 134:601–606.15. Russell PJ, Raghavan D, Gregory P, Philips J, Wills EJ, Jelbart M, et al. Bladder cancer xenografts: a model of tumor cell heterogeneity. Cancer Res. 1986. 46(4 Pt 2):2035–2040.16. Ratliff TL, Gillen D, Catalona WJ. Requirement of a thymus dependent immune response for BCG-mediated antitumor activity. J Urol. 1987. 137:155–158.17. Hudson MA, Ritchey JK, Catalona WJ, Brown EJ, Ratliff TL. Comparison of the fibronectin-binding ability and antitumor efficacy of various mycobacteria. Cancer Res. 1990. 50:3843–3847.18. Lee KE, Weiss GH, O'Donnell RW, Cockett AT. Reduction of bladder cancer growth in mice treated with intravesical Bacillus Calmette Guerin and systemic interleukin 2. J Urol. 1987. 137:1270–1273.19. Lee SJ, Chung CE, Kim SW, Lee CB, Kang SH, Cho YH, et al. Efficacy of paclitaxel-loaded bioadhesive drug deliver system based on glyceryl monooleate nanoparticle in an orthotopic murine bladder cancer model. Korean J Urol. 2004. 45:817–822.20. Erturk E, Cohen SM, Price JM, Bryan GT. Pathogenesis, histology, and transplantability of urinary bladder carcinomas induced in albino rats by oral administration of N-(4-(5-nitro-2-furyl)-2-thiazolyl)formamide. Cancer Res. 1969. 29:2219–2228.21. Erturk E, Price JM, Morris JE, Cohen S, Leith RS, Von Esch AM, et al. The production of carcinoma of the urinary bladder in rats by feeding N-[3-(5-nitro-2-furyl)-2-thiazolyl]formamide. Cancer Res. 1967. 27:1998–2002.22. Herman CJ, Vegt PD, Debruyne FM, Vooijs GP, Ramaekers FC. Squamous and transitional elements in rat bladder carcinomas induced by N-butyl-N-4-hydroxybutyl-nitrosamine (BBN). A study of cytokeratin expression. Am J Pathol. 1985. 120:419–426.23. Oyasu R, Samma S, Ozono S, Bauer K, Wallemark CB, Homma Y. Induction of high-grade, high-stage carcinomas in the rat urinary bladder. Cancer. 1987. 59:451–458.24. Gunther JH, Jurczok A, Wulf T, Brandau S, Deinert I, Jocham D, et al. Optimizing syngeneic orthotopic murine bladder cancer (MB49). Cancer Res. 1999. 59:2834–2837.25. Parsons CL, Stauffer C, Schmidt JD. Impairment of antibacterial effect of bladder surface mucin by protamine sulfate. J Infect Dis. 1981. 144:180.26. Sindhwani P, Hampton JA, Baig MM, Keck R, Selman SH. Curcumin prevents intravesical tumor implantation of the MBT-2 tumor cell line in C3H mice. J Urol. 2001. 166:1498–1501.27. Chin J, Kadhim S, Garcia B, Kim YS, Karlik S. Magnetic resonance imaging for detecting and treatment monitoring of orthotopic murine bladder tumor implants. J Urol. 1991. 145:1297–1301.28. Satoh H, Morimoto Y, Arai T, Asanuma H, Kawauchi S, Seguchi K, et al. Intravesical ultrasonography for tumor staging in an orthotopically implanted rat model of bladder cancer. J Urol. 2007. 177:1169–1173.29. Siemens DR, Austin JC, See WA, Tartaglia J, Ratliff TL. Evaluation of gene transfer efficiency by viral vectors to murine bladder epithelium. J Urol. 2001. 165:667–671.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Paclitaxel-Loaded Bioadhesive Drug Deliver System Based on Glyceryl Monooleate Nanoparticle in an Orthotopic Murine Bladder Cancer Model
- Altered Biological Potential and Radioresponse of Murine Tumors in Different Microenvironments
- Delayed Spontaneous Rupture of an Orthotopic Ileal Neobladder
- Animal Models of Cancer in the Head and Neck Region
- Radical Cystectomy and Orthotopic Bladder Substitution Using Ileum