J Korean Med Sci.
2015 Mar;30(3):233-239. 10.3346/jkms.2015.30.3.233.
Eupatilin Ameliorates Collagen Induced Arthritis
- Affiliations
-
- 1Clinical Immunology and STEM (CiSTEM) Cell Laboratory, Convergent Research Consortium for Immunologic Disease, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. juji@catholic.ac.kr
- 2Division of Rheumatology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2133298
- DOI: http://doi.org/10.3346/jkms.2015.30.3.233
Abstract
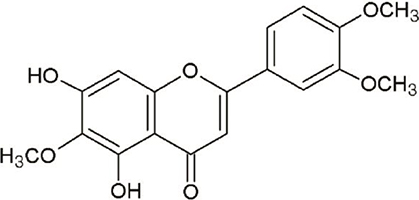
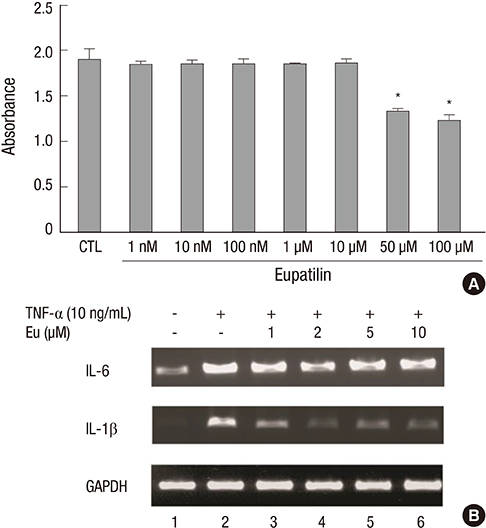
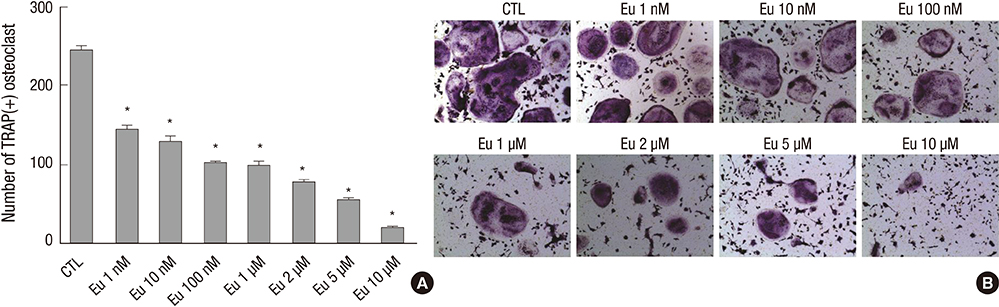
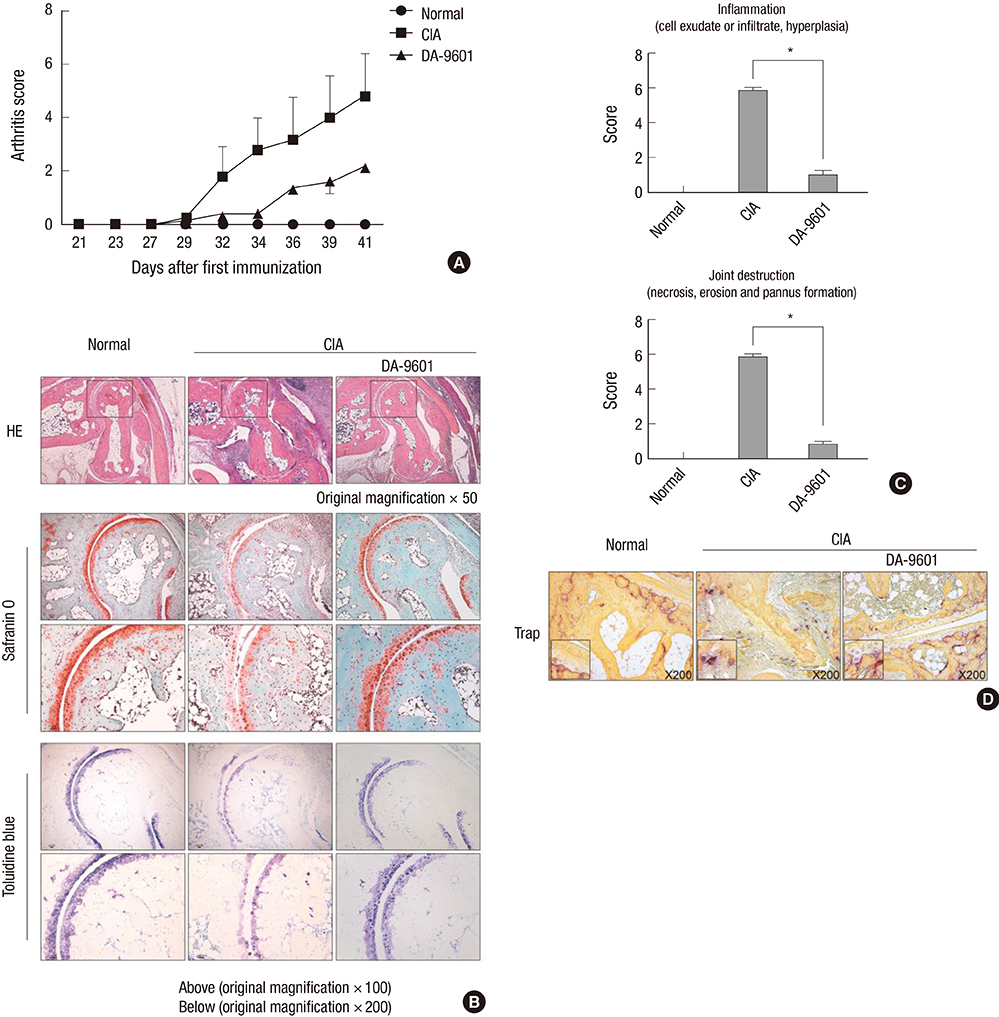
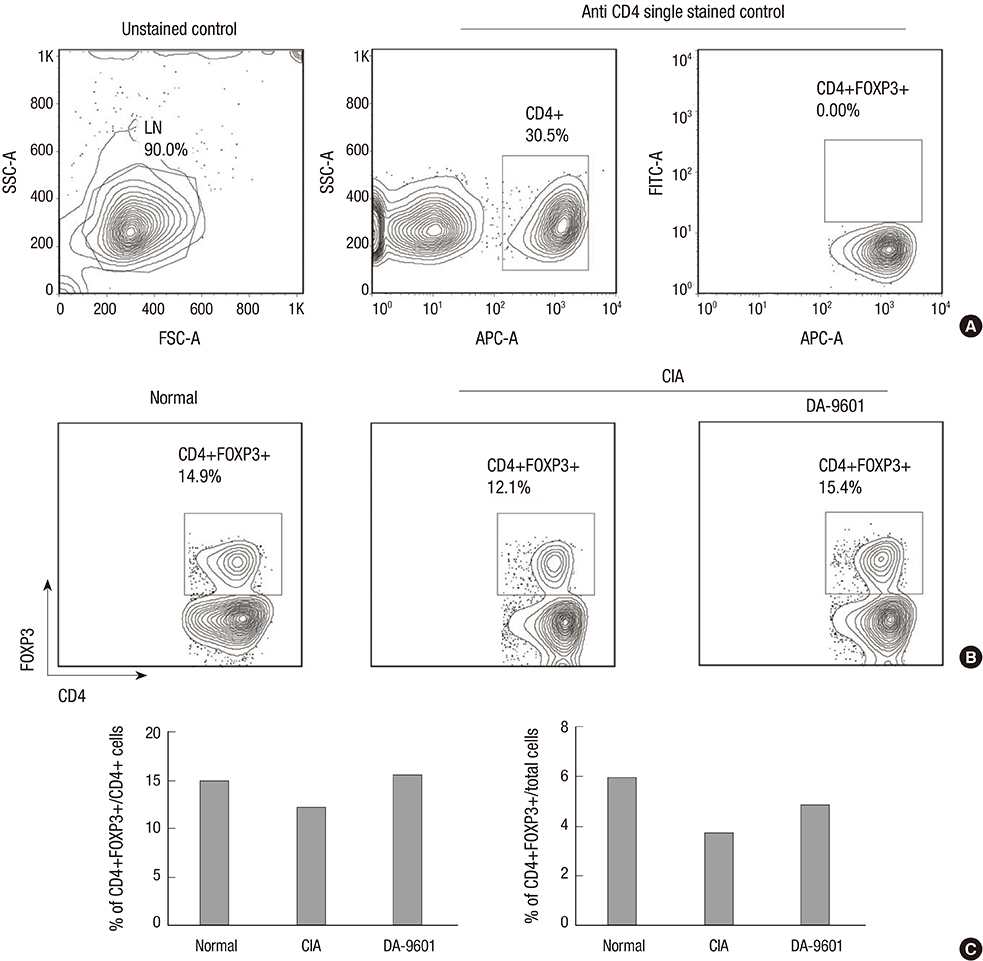
- Eupatilin is the main active component of DA-9601, an extract from Artemisia. Recently, eupatilin was reported to have anti-inflammatory properties. We investigated the anti-arthritic effect of eupatilin in a murine arthritis model and human rheumatoid synoviocytes. DA-9601 was injected into collagen-induced arthritis (CIA) mice. Arthritis score was regularly evaluated. Mouse monocytes were differentiated into osteoclasts when eupatilin was added simultaneously. Osteoclasts were stained with tartrate-resistant acid phosphatase and then manually counted. Rheumatoid synoviocytes were stimulated with TNF-alpha and then treated with eupatilin, and the levels of IL-6 and IL-1beta mRNA expression in synoviocytes were measured by RT-PCR. Intraperitoneal injection of DA-9601 reduced arthritis scores in CIA mice. TNF-alpha treatment of synoviocytes increased the expression of IL-6 and IL-1beta mRNAs, which was inhibited by eupatilin. Eupatilin decreased the number of osteoclasts in a concentration dependent manner. These findings, showing that eupatilin and DA-9601 inhibited the expression of inflammatory cytokines and the differentiation of osteoclasts, suggest that eupatilin and DA-9601 is a candidate anti-inflammatory agent.
MeSH Terms
-
Animals
Anti-Inflammatory Agents/pharmacology/*therapeutic use
Arthritis, Experimental/chemically induced/*drug therapy
Arthritis, Rheumatoid/drug therapy/pathology
Cell Differentiation/*drug effects
Cells, Cultured
Collagen Type II
Cytokines/biosynthesis
Disease Models, Animal
Drugs, Chinese Herbal/therapeutic use
Female
Flavonoids/pharmacology/*therapeutic use
Humans
Inflammation/drug therapy/immunology
Interleukin-1beta/genetics/metabolism
Interleukin-6/genetics/metabolism
Lymph Nodes/cytology
Mice
Mice, Inbred DBA
Monocytes/cytology
Osteoclasts/*cytology
Plant Extracts/pharmacology
RNA, Messenger/biosynthesis
Synovial Membrane/cytology
T-Lymphocytes, Regulatory/cytology/immunology
Tumor Necrosis Factor-alpha/pharmacology
Anti-Inflammatory Agents
Collagen Type II
Cytokines
Drugs, Chinese Herbal
Flavonoids
Interleukin-1beta
Interleukin-6
Plant Extracts
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Topical Application of Eupatilin Ameliorates Atopic Dermatitis-Like Skin Lesions in NC/Nga Mice
Ji Hyun Lee, Ye Jin Lee, Jun Young Lee, Young Min Park
Ann Dermatol. 2017;29(1):61-68. doi: 10.5021/ad.2017.29.1.61.
Reference
-
1. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003; 423:356–361.2. van Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol. 2009; 5:531–541.3. Choy EH, Kavanaugh AF, Jones SA. The problem of choice: current biologic agents and future prospects in RA. Nat Rev Rheumatol. 2013; 9:154–163.4. Khanna D, Sethi G, Ahn KS, Pandey MK, Kunnumakkara AB, Sung B, Aggarwal A, Aggarwal BB. Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol. 2007; 7:344–351.5. Morinobu A, Biao W, Tanaka S, Horiuchi M, Jun L, Tsuji G, Sakai Y, Kurosaka M, Kumagai S. (-)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008; 58:2012–2018.6. Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, Xu D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis. 2012; 71:129–135.7. Choi SC, Choi EJ, Oh HM, Lee S, Lee JK, Lee MS, Shin YI, Choi SJ, Chae JR, Lee KM, et al. DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-alpha-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-kappaB pathways in human gastric epithelial cells. World J Gastroenterol. 2006; 12:4850–4858.8. Seol SY, Kim MH, Ryu JS, Choi MG, Shin DW, Ahn BO. DA-9601 for erosive gastritis: results of a double-blind placebo-controlled phase III clinical trial. World J Gastroenterol. 2004; 10:2379–2382.9. Oh TY, Ahn GJ, Choi SM, Ahn BO, Kim WB. Increased susceptibility of ethanol-treated gastric mucosa to naproxen and its inhibition by DA-9601, an Artemisia asiatica extract. World J Gastroenterol. 2005; 11:7450–7456.10. Park BB, Yoon J, Kim E, Choi J, Won Y, Choi J, Lee YY. Inhibitory effects of eupatilin on tumor invasion of human gastric cancer MKN-1 cells. Tumour Biol. 2013; 34:875–885.11. Choi EJ, Oh HM, Na BR, Ramesh TP, Lee HJ, Choi CS, Choi SC, Oh TY, Choi SJ, Chae JR, et al. Eupatilin protects gastric epithelial cells from oxidative damage and down-regulates genes responsible for the cellular oxidative stress. Pharm Res. 2008; 25:1355–1364.12. Choi EJ, Lee S, Chae JR, Lee HS, Jun CD, Kim SH. Eupatilin inhibits lipopolysaccharide-induced expression of inflammatory mediators in macrophages. Life Sci. 2011; 88:1121–1126.13. Lee SH, Bae EA, Park EK, Shin YW, Baek NI, Han EJ, Chung HG, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps in IgE-induced hypersensitivity. Int Immunopharmacol. 2007; 7:1678–1684.14. Kim YD, Choi SC, Oh TY, Chun JS, Jun CD. Eupatilin inhibits T-cell activation by modulation of intracellular calcium flux and NF-kappaB and NF-AT activity. J Cell Biochem. 2009; 108:225–236.15. Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010; 233:233–255.16. Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008; 118:3537–3545.17. Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013; 9:24–33.18. Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009; 5:667–676.19. Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg LO, Kan YW, Chan K, Hassenpflug J, Freitag-Wolf S, Varoga D, Lippross S, et al. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum Dis. 2011; 70:844–850.20. Yang H, Lee DY, Jeon M, Suh Y, Sung SH. Determination of five active compounds in Artemisia princeps and A. capillaris based on UPLC-DAD and discrimination of two species with multivariate analysis. Arch Pharm Res. 2014; 37:617–625.21. Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989; 83:585–592.22. Yeon JT, Kim KJ, Choi SW, Moon SH, Park YS, Ryu BJ, Oh J, Kim MS, Erkhembaatar M, Son YJ, et al. Anti-osteoclastogenic activity of praeruptorin A via inhibition of p38/Akt-c-Fos-NFATc1 signaling and PLCgamma-independent Ca2+ oscillation. PLoS One. 2014; 9:e88974.23. Ichikawa H, Aggarwal BB. Guggulsterone inhibits osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand and by tumor cells by suppressing nuclear factor-kappaB activation. Clin Cancer Res. 2006; 12:662–668.24. Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem. 2011; 286:11492–11505.25. Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007; 2:1269–1275.26. Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006; 24:677–688.27. Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008; 112:1557–1569.28. Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004; 4:889–899.29. Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007; 8:457–462.30. Wong CP, Nguyen LP, Noh SK, Bray TM, Bruno RS, Ho E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol Lett. 2011; 139:7–13.31. Ahmad SF, Zoheir KM, Abdel-Hamied HE, Ashour AE, Bakheet SA, Attia SM, Abd-Allah AR. Grape seed proanthocyanidin extract has potent anti-arthritic effects on collagen-induced arthritis by modifying the T cell balance. Int Immunopharmacol. 2013; 17:79–87.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eupatilin treatment inhibits transforming growth factor beta-induced endometrial fibrosis in vitro
- Effects of Eupatilin on the Release of Leukotriene B4 , by Helicobacter pylori - stimulated Neutrophils and Gastric Mucosal Cells
- Topical Application of Eupatilin Ameliorates Atopic Dermatitis-Like Skin Lesions in NC/Nga Mice
- The Effect of a High Dose of Methotrexate on Type II Collagen induced Arthritis in Rats
- The hepato-protective effect of eupatilin on an alcoholic liver disease model of rats