Korean J Urol.
2015 Mar;56(3):197-204. 10.4111/kju.2015.56.3.197.
Optimizing in vivo gene transfer into mouse corpus cavernosum by use of surface electroporation
- Affiliations
-
- 1National Research Center for Sexual Medicine and Department of Urology, Inha University School of Medicine, Incheon, Korea. jksuh@inha.ac.kr
- 2Department of Urology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- KMID: 2133287
- DOI: http://doi.org/10.4111/kju.2015.56.3.197
Abstract
- PURPOSE
Electroporation is known to enhance the efficiency of gene transfer through a transient increase in cell membrane permeability. The aim of this study was to determine the optimal conditions for in vivo electroporation-mediated gene delivery into mouse corpus cavernosum.
MATERIALS AND METHODS
Diabetes was induced in C57BL/6 mice by intraperitoneal injections of streptozotocin. After intracavernous injection of pCMV-Luc (100 microg/40 microL), different electroporation settings (5-50 V, 8-16 pulses with a duration of 40-100 ms) were applied to the penis to establish the optimal conditions for electroporation. Gene expression was evaluated by luciferase assay. We also assessed the undesired consequences of electroporation by visual inspection and hematoxylin-eosin staining of penile tissue.
RESULTS
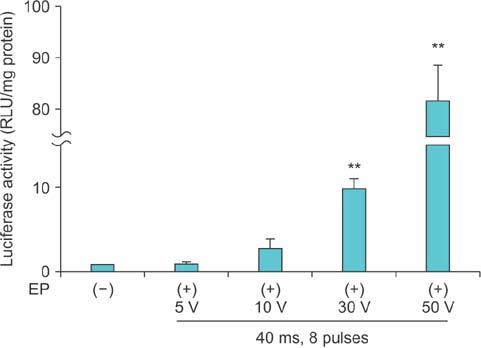
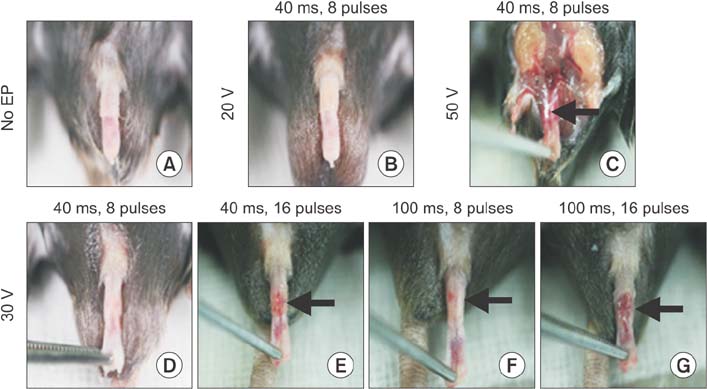
Electroporation profoundly induced gene expression in the corpus cavernosum tissue of normal mice in a voltage-dependent manner. We observed electrical burn scars in the penis of normal mice who received electroporation with eight 40-ms pulses at a voltage of 50 V and sixteen 40-ms pulses, eight 100-ms pulses, and sixteen 100-ms pulses at a voltage of 30 V. No detectable burn scars were noted in normal mice stimulated with eight 40-ms pulses at a voltage of 30 V. Electroporation also significantly induced gene expression in diabetic mice stimulated with 40-ms pulse at a voltage of 30 V without injury to the penis.
CONCLUSIONS
We have established the optimal electroporation conditions for maximizing gene transfer into the corpus cavernosum of mice while avoiding damage to the erectile tissue. The electroporation-mediated gene delivery technique will be a valuable tool for gene therapy in the field of erectile dysfunction.
Keyword
MeSH Terms
-
Animals
Diabetes Mellitus, Experimental/complications
Electroporation/*methods
Erectile Dysfunction/*therapy
Gene Expression
Gene Transfer Techniques
Genes, Reporter
Genetic Therapy/*methods
Luciferases/metabolism
Male
Mice
Mice, Inbred C57BL
Penile Erection/physiology
Penis/*physiopathology
Transfection
Luciferases
Figure
Reference
-
1. Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Sildenafil Study Group. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998; 338:1397–1404.2. Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil Diabetes Study Group. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. JAMA. 1999; 281:421–426.3. Choi HK, Kim JJ, Kim SC, Suh J, Park YK, Choi S, et al. A randomised, double-blind, parallel, placebo-controlled study of the efficacy and safety of tadalafil administered on-demand to men with erectile dysfunction in Korea. Korean J Urol. 2006; 47:852–858.4. Martinez-Jabaloyas JM, Gil-Salom M, Villamon-Fort R, Pastor-Hernandez F, Martinez-Garcia R, Garcia-Sisamon F. Prognostic factors for response to sildenafil in patients with erectile dysfunction. Eur Urol. 2001; 40:641–646.5. Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil: review of the literature. Eur J Med Res. 2002; 7:435–446.6. Garban H, Marquez D, Magee T, Moody J, Rajavashisth T, Rodriguez JA, et al. Cloning of rat and human inducible penile nitric oxide synthase. Application for gene therapy of erectile dysfunction. Biol Reprod. 1997; 56:954–963.7. Christ GJ, Rehman J, Day N, Salkoff L, Valcic M, Melman A, et al. Intracorporal injection of hSlo cDNA in rats produces physiologically relevant alterations in penile function. Am J Physiol. 1998; 275(2 Pt 2):H600–H608.8. Champion HC, Bivalacqua TJ, Hyman AL, Ignarro LJ, Hellstrom WJ, Kadowitz PJ. Gene transfer of endothelial nitric oxide synthase to the penis augments erectile responses in the aged rat. Proc Natl Acad Sci U S A. 1999; 96:11648–11652.9. Harraz A, Shindel AW, Lue TF. Emerging gene and stem cell therapies for the treatment of erectile dysfunction. Nat Rev Urol. 2010; 7:143–152.10. Henry TD, Hirsch AT, Goldman J, Wang YL, Lips DL, McMillan WD, et al. Safety of a non-viral plasmid-encoding dual isoforms of hepatocyte growth factor in critical limb ischemia patients: a phase I study. Gene Ther. 2011; 18:788–794.11. Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin Infect Dis. 2011; 53:296–302.12. Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006; 208:299–318.13. Campos SK, Barry MA. Current advances and future challenges in Adenoviral vector biology and targeting. Curr Gene Ther. 2007; 7:189–204.14. Wells DJ. Electroporation and ultrasound enhanced non-viral gene delivery in vitro and in vivo. Cell Biol Toxicol. 2010; 26:21–28.15. Andreason GL, Evans GA. Introduction and expression of DNA molecules in eukaryotic cells by electroporation. Biotechniques. 1988; 6:650–660.16. Somiari S, Glasspool-Malone J, Drabick JJ, Gilbert RA, Heller R, Jaroszeski MJ, et al. Theory and in vivo application of electroporative gene delivery. Mol Ther. 2000; 2:178–187.17. Mir LM, Moller PH, Andre F, Gehl J. Electric pulse-mediated gene delivery to various animal tissues. Adv Genet. 2005; 54:83–114.18. Satkauskas S, Ruzgys P, Venslauskas MS. Towards the mechanisms for efficient gene transfer into cells and tissues by means of cell electroporation. Expert Opin Biol Ther. 2012; 12:275–286.19. Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008; 26:5896–5903.20. Yang FQ, Yu YY, Wang GQ, Chen J, Li JH, Li YQ, et al. A pilot randomized controlled trial of dual-plasmid HBV DNA vaccine mediated by in vivo electroporation in chronic hepatitis B patients under lamivudine chemotherapy. J Viral Hepat. 2012; 19:581–593.21. Magee TR, Ferrini M, Garban HJ, Vernet D, Mitani K, Rajfer J, et al. Gene therapy of erectile dysfunction in the rat with penile neuronal nitric oxide synthase. Biol Reprod. 2002; 67:20–28.22. Roche JA, Ford-Speelman DL, Ru LW, Densmore AL, Roche R, Reed PW, et al. Physiological and histological changes in skeletal muscle following in vivo gene transfer by electroporation. Am J Physiol Cell Physiol. 2011; 301:C1239–C1250.23. Lee M, Ryu JK, Oh SM, Lee E, Shin HY, Song SU, et al. Water-soluble lipopolymer as a gene carrier to corpus cavernosum. Int J Impot Res. 2005; 17:326–334.24. Jin HR, Kim WJ, Song JS, Choi MJ, Piao S, Shin SH, et al. Functional and morphologic characterizations of the diabetic mouse corpus cavernosum: comparison of a multiple low-dose and a single high-dose streptozotocin protocols. J Sex Med. 2009; 6:3289–3304.25. Vicat JM, Boisseau S, Jourdes P, Laine M, Wion D, Bouali-Benazzouz R, et al. Muscle transfection by electroporation with high-voltage and short-pulse currents provides high-level and long-lasting gene expression. Hum Gene Ther. 2000; 11:909–916.26. Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci U S A. 1999; 96:4262–4267.27. Ryu JK, Suh JK. Therapeutic angiogenesis as a potential future treatment strategy for erectile dysfunction. World J Mens Health. 2012; 30:93–98.28. Ouma GO, Rodriguez E, Muthumani K, Weiner DB, Wilensky RL, Mohler ER 3rd. In vivo electroporation of constitutively expressed HIF-1α plasmid DNA improves neovascularization in a mouse model of limb ischemia. J Vasc Surg. 2014; 59:786–793.29. Ferraro B, Cruz YL, Baldwin M, Coppola D, Heller R. Increased perfusion and angiogenesis in a hindlimb ischemia model with plasmid FGF-2 delivered by noninvasive electroporation. Gene Ther. 2010; 17:763–769.30. Makarevich P, Tsokolaeva Z, Shevelev A, Rybalkin I, Shevchenko E, Beloglazova I, et al. Combined transfer of human VEGF165 and HGF genes renders potent angiogenic effect in ischemic skeletal muscle. PLoS One. 2012; 7:e38776.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacologic Effect of Phentolamine on Norepinephrine Induced Contraction of Corpus Cavernosum
- A Case or Priapism Treated by Corpus Cavernosum-Saphenous Vein Anastomosis
- Marker Gene Transfer into Cells of Rat Achilles Tendon by In Vivo Electroporation
- A Fibrotic Nodule in the Corpus Cavernosum
- The Effect of Nitric Oxide on Cat Corpus Cavernosum Relaxation Under Hypoxia (In vivo Study)