J Korean Soc Endocrinol.
2005 Aug;20(4):362-374. 10.3803/jkes.2005.20.4.362.
Chromosomal Analysis of Anaplastic Thyroid Carcinomas by Comparative Genomic Hybridization
- Affiliations
-
- 1Department of Internal Medicine, Wallace Memorial Baptist Hospital, USA.
- 2Department of Internal Medicine, School of Medicine, Pusan National university, Busan, Korea.
- 3Department of Pathology, School of Medicine, Pusan National university, Busan, Korea.
- 4Department of Pathology University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- KMID: 2100467
- DOI: http://doi.org/10.3803/jkes.2005.20.4.362
Abstract
-
BACKGROUND: Compared with common well-differentiated thyroid carcinomas, the genetic alterations underlying the development and progression of anaplastic thyroid carcinomas(ATC) are still uncharacterized. Comparative genomic hybridization(CGH) is a cytogenetic technique that can identify gains and losses in the DNA sequence copy number in tumors.
METHODS
The authors studied the changes in the DNA copy number due to CGH in paraffin-embedded tissue blocks of 17 ATC cases, and tried to ascertain whether the genomic changes correlate with the clinicopathological parameters including patients' age, sex, primary tumor size, lymphovascular invasion, extrathyroid extension, regional node metastasis and immunohistochemical expression of cyclin D1.
RESULTS
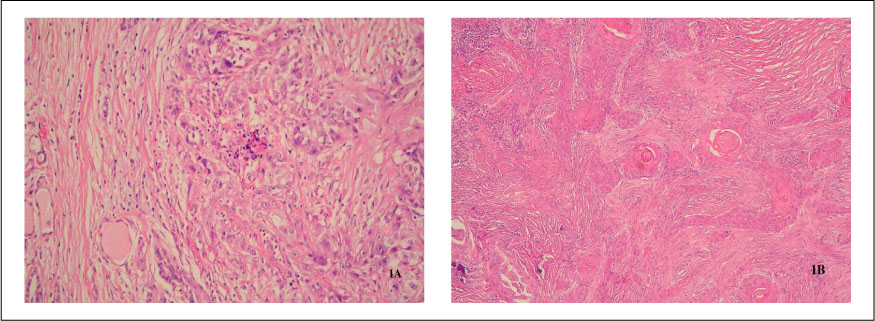
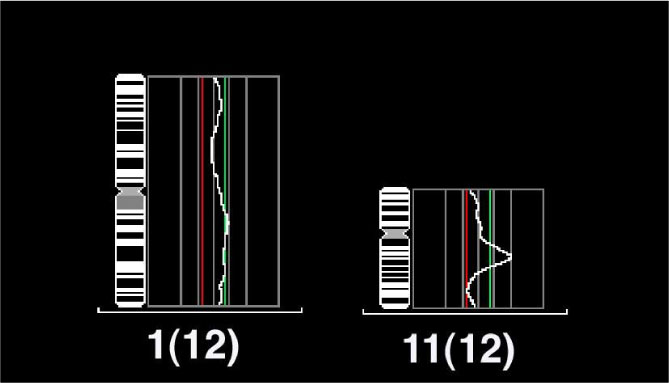
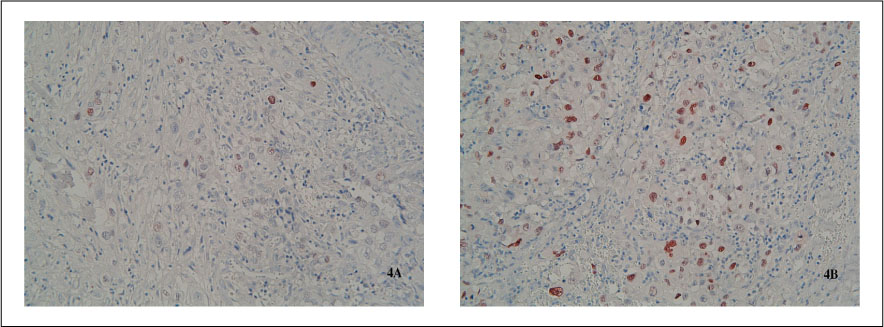
Fourteen of the 17 samples(82.4%) showed chromosomal changes, with a mean number of gains or losses per carcinoma of 3.6(range 2~6; 30 gains and 21 losses). The most frequently detected imbalance was the gain of chromosome 1q, which was seen in 35.7% of cases, particularly commonly in ATC associated with a papillary thyroid carcinoma. Other commonly occurring gains were present in 11q13 and 19(28.6%, respectively). Genomic amplification was detected in all four cases showing the 11q13 gain. Genomic losses were commonly noted in 3q, 6q, 18q andchi(21.4%, respectively). When numerical CGH alterations were compared to the clinicopathological parameters, there were no significant correlations(P>0.05). Cyclin D1 expression was noted in sixteen of the 17 cases(94.1%), but the extent of cyclin D1 expression was not correlated with the numerical CGH alterations(P>0.05).
CONCLUSION
Taken together, the aberrations of 1q, 3q, 6q, 11q13 and 18q are relatively common in ATC, and may play an important role it developement. These findings should lead to the characterization of tumor suppressor genes and oncogenes that are potentially involved in the carcinogenesis of ATC. The amplification of 11q13 is characteristically found, but cyclin D1 in this region may be innocent of the aggressiveness of these carcinomas.
MeSH Terms
Figure
Reference
-
1. Tan RK, Finley RK 3rd, Driscoll D, Bakamjian V, Hicks WL Jr, Shedd DP. Anaplastic carcinoma of the thyroid: a 24-year experience. Head Neck. 1995. 17:41–48.2. Wynford-Thomas D. Origin and progression of thyroid ithelial tumours: cellular and molecular mechanisms. Horm Res. 1997. 47:145–157.3. Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990. 66:321–330.4. Boltze C, Roessner A, Landt O, Szibor R, Peters B, Schneider-Stock R. Homozygous proline at codon 72 of p53 as a potential risk factor favoring the development of differentiated thyroid carcinoma. Int J Oncol. 2002. 21:1151–1154.5. Garcia-Rostan G, Tallini G, Herrero A, D'Aquila TG, Carcangiu ML, Rimm D. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res. 1999. 59:1811–1815.6. Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998. 396:643–649.7. Pierotti MA, Bongarzone I, Borello MG, Greco A, Pilotti S, Sozzi G. Cytogenetics and molecular genetics of carcinomas arising from thyroid epithelial follicular cells. Genes Chromosomes Cancer. 1996. 16:1–14.8. Bauer AJ, Cavalli LR, Rone JD, Francis GL, Burch HB, Tuttle RM, Ringel MD, Stratakis CA, Haddad BR. Evaluation of adult papillary thyroid carcinomas by comparative genomic hybridization and microsatellite instability analysis. Cancer Genet Cytogenet. 2002. 135:182–186.9. Stoler DL, Datta RV, Charles MA, Block AW, Brenner BM, Sieczka EM, Hicks WL Jr, Loree TR, Anderson G. Genomic instability measurement in the diagnosis of thyroid neoplasms. Head Neck. 2002. 24:290–295.10. Ried T, Heselmeyer-Haddad K, Blegen H, Schrock E, Auer G. Genomic changes defining the genesis, progression, and malignancy potential in solid human tumors: a phenotype/genotype correlation. Genes Chromosomes Cancer. 1999. 25:195–204.11. Kallioniemi A, Kallioniemi OP, Sudar D, Rutovitz D, Gray JW, Waldman F, Pinkel D. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science. 1992. 258:818–821.12. Wilkens L, Tchinda J, Burkhardt D, Nolte M, Werner M, Georgii A. Analysis of hematologic diseases using conventional karyotyping, fluorescence in situ hybridization (FISH), and comparative genomic hybridization (CGH). Hum Pathol. 1998. 29:833–839.13. Kuukasjarvi T, Tanner M, Pennanen S, Karhu R, Visakorpi T, Isola J. Optimizing DOP-PCR for universal amplification of small DNA samples in comparative genomic hybridization. Genes Chromosomes Cancer. 1997. 18:94–101.14. Fagin JA. Genetic basis of endocrine disease 3: Molecular defects in thyroid gland neoplasia. J Clin Endocrinol Metab. 1992. 75:1398–1400.15. Hemmer S, Wasenius VM, Knuutila S, Franssila K, Joensuu H. DNA copy number changes in thyroid carcinoma. Am J Pathol. 1999. 154:1539–1547.16. Wilkens L, Benten D, Tchinda J, Brabant G, Potter E, Dralle H, von Wasielewski R. Aberrations of chromosomes 5 and 8 as recurrent cytogenetic events in anaplastic carcinoma of the thyroid as detected by fluorescence in situ hybridisation and comparative genomic hybridisation. Virchows Arch. 2000. 436:312–318.17. Wreesmann VB, Ghossein RA, Patel SG, Harris CP, Schnaser EA, Shaha AR, Tuttle RM, Shah JP, Rao PH, Singh B. Genome-wide appraisal of thyroid cancer progression. Am J Pathol. 2002. 161:1549–1556.18. Rodrigues RF, Roque L, Rosa-Santos J, Cid O, Soares J. Chromosomal imbalances associated with anaplastic transformation of follicular thyroid carcinomas. Br J Cancer. 2004. 90:492–496.19. Zhu XL, Hartwick W, Rohan T, Kandel R. Cyclin D1 gene amplification and protein expression in benign breast disease and breast carcinoma. Mod Pathol. 1998. 11:1082–1088.20. Smith BD, Haffty BG, Sasaki CT. Molecular markers in head and neck squamous cell carcinoma: their biological function and prognostic significance. Ann Otol Rhinol Laryngol. 2001. 110:221–228.21. Tut VM, Braithwaite KL, Angus B, Neal DE, Lunec J, Mellon JK. Cyclin D1 expression in transitional cell carcinoma of the bladder: correlation with p53, waf1, pRb and Ki67. Br J Cancer. 2001. 84:270–275.22. Wang S, Lloyd RV, Hutzler MJ, Safran MS, Patwardhan NA, Khan A. The role of cell cycle regulatory protein, cyclin D1, in the progression of thyroid cancer. Mod Pathol. 2000. 13:882–887.23. Rosai J, Carcangiu ML, DeLellis RA. Tumors of the thyroid gland. 1992. Washington, DC: Armed Forces Institute of Pathology.24. Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A laboratory manual. 1989. 2nd ed. New York: Cold Spring Harbor Laboratory Press.25. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000. 100:57–70.26. Jenkins RB, Hay ID, Herath JF, Schultz CG, Spurbeck JL, Grant CS, Goellner JR, Dewald GW. Frequent occurrence of cytogenetic abnormalities in sporadic nonmedullary thyroid carcinoma. Cancer. 1990. 66:1213–1220.27. Isola JJ, de Vries S, Chu LW, Ghazvini S, Waldman FM. Analysis of changes in DNA sequence copy number by comparative genomic hybridization in archival paraffin-embedded tumor samples. Am J Pathol. 1994. 145:1301–1308.28. Miura D, Wada N, Chin K, Magrane GG, Wong M, Duh QY, Clark OH. Anaplastic thyroid cancer: cytogenetic patterns by comparative genomic hybridization. Thyroid. 2003. 13:283–290.29. Komoike Y, Tamaki Y, Sakita I, Tomita N, Ohue M, Sekimoto M, Miyazaki M, Kadota M, Masuda N, Ooka M, Ohnishi T, Nakano Y, Kozaki T, Kobayashi T, Matsuura N, Ikeda T, Horii A, Monden M. Comparative genomic hybridization defines frequent loss on 16p in human anaplastic thyroid carcinoma. Int J Oncol. 1999. 14:1157–1162.30. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990. 61:759–767.31. Zitzelsberger H, Lehmann L, Hieber L, Weier HU, Janish C, Fung J, Negele T, Spelsberg F, Lengfelder E, Demidchik EP, Salassidis K, Kellerer AM, Werner M, Bauchinger M. Cytogenetic changes in radiation-induced tumors of the thyroid. Cancer Res. 1999. 59:135–140.32. Bjrkqvist AM, Tammilehto L, Anttila S, Mattson K, Knuutila S. Recurrent DNA copy number changes in 1q, 4q, 6q, 9p, 13q, 14q and 22q detected by comparative genomic hybridization in malignant mesothelioma. Br J Cancer. 1997. 75:523–527.33. Kallioniemi A, Kallioniemi OP, Citro G, Sauter G, DeVries S, Kerschmann R, Caroll P, Waldman F. Identification of gains and losses of DNA sequences in primary bladder cancer by comparative genomic hybridization. Genes Chromosomes Cancer. 1995. 12:213–219.34. Monni O, Joensuu H, Franssila K, Knuutila S. DNA copy number changes in diffuse large B-cell lymphoma-comparative genomic hybridization study. Blood. 1996. 87:5269–5278.35. Mark J, Ekedahl C, Dahlenfors R, Westermark B. Cytogenetical observations in five human anaplastic thyroid carcinomas. Hereditas. 1987. 107:163–174.36. Kjellman M, Kallioniemi OP, Karhu R, Hoog A, Farnebo LO, Auer G, Larsson C, Backdahl M. Genetic aberrations in adrenocortical tumors detected using comparative genomic hybridization correlate with tumor size and malignancy. Cancer Res. 1996. 56:4219–4223.37. Ried T, Heselmeyer-Haddad K, Blegen H, Schrock E, Auer G. Genomic changes defining the genesis, progression, and malignancy potential in solid human tumors: a phenotype/genotype correlation. Genes Chromosomes Cancer. 1999. 25:195–204.38. Hemmer S, Wasenius VM, Knuutila S, Joensuu H, Franssila K. Comparison of benign and malignant follicular thyroid tumours by comparative genomic hybridization. Br J Cancer. 1998. 78:1012–1017.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Chromosomal Aberrations in Thyroid Papillary Carcinomas Using Comparative Genomic Hybridization (CGH)
- Genetic Alterations in Colorectal Carcinoma Detected by Comparative Genomic Hybridization
- Chromosomal gains and losses in primary ovarian carcinomas by comparative genomic hybridization
- Genetic Alterations in Gastric Carcinomas and Adjacent Mucosa Detected by Comparative Genomic Hybridization (CGH)
- Analysis of Chromosomal Aberrations in Lung Cancer Cell Line, NCI-H1373