Korean J Pain.
2011 Dec;24(4):191-198. 10.3344/kjp.2011.24.4.191.
Effects of Low and High Molecular Weight Hyaluronic Acids on Peridural Fibrosis and Inflammation in Lumbar Laminectomized Rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Anesthesiology and Pain Medicine, Konkuk University School of Medicine, Seoul, Korea.
- 3Department of Anesthesiology and Pain Medicine, Jeju University School of Medicine, Jeju, Korea.
- 4Department of Clinical Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Anesthesiology and Pain Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. anesthe@skku.edu
- KMID: 2074013
- DOI: http://doi.org/10.3344/kjp.2011.24.4.191
Abstract
- BACKGROUND
Postlaminectomy peridural fibrosis is inevitable. Some studies have compared and identified the effects of high molecular weight hyaluronic acids (HMWHA) and low molecular weight hyaluronic acids (LMWHA) on peridural fibrosis in postlaminectomy animal models. However, no studies have been found that compare pain behaviors between hyaluronic acids or among hyaluronic acids and other solid materials. The purpose of this study was to examine the correlation between pain-related behaviors and histopathologic changes in laminectomized rats using various peridurally administered materials.
METHODS
Forty male Sprague-Dawley rats, laminectomized at the L5 and L6 levels, were divided into four groups: group C, laminectomy only; group L, laminectomy and LMWHA application; group H, laminectomy and HMWHA application; group F, laminectomy and fat interposition. Pain behaviors were checked before, 3 days, 1 week, and 3 weeks after surgery. Histopathological changes were checked at the L5 level 3 weeks after the surgery.
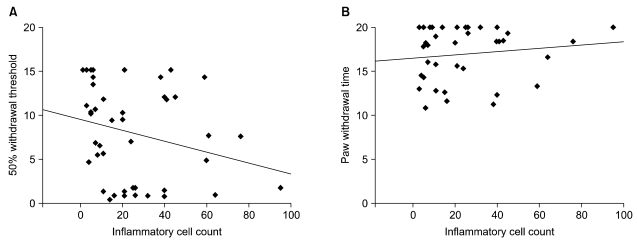
RESULTS
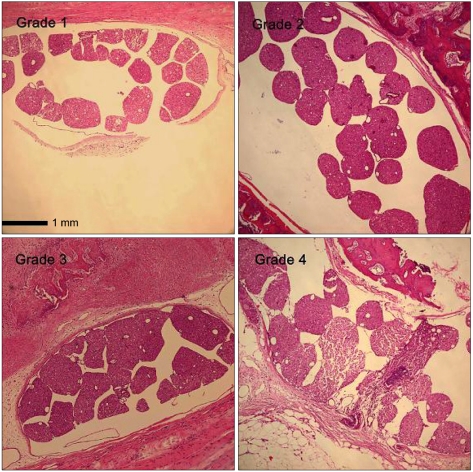
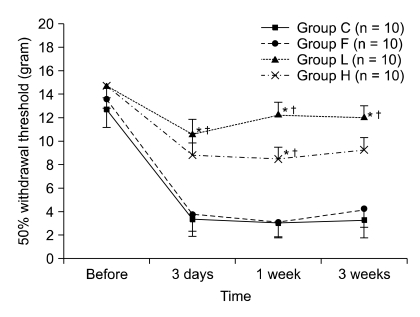
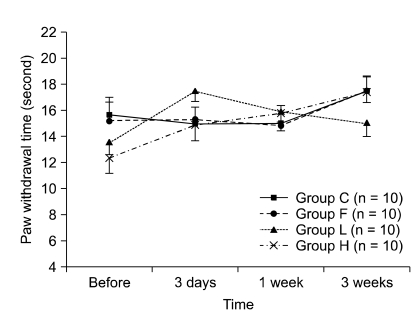
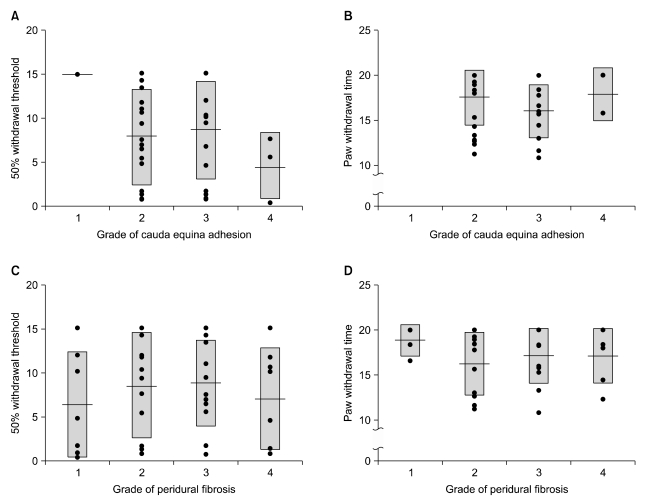
The 50% withdrawal thresholds in groups L and H were higher than that in groups C and F three days after laminectomy (P < 0.05). The paw withdrawal time did not change among the groups and in each group during the study period. Peridural fibrosis in group F was significantly lower than in the other groups (P < 0.05).
CONCLUSIONS
Hyaluronic acids significantly reduced mechanical allodynia but not thermal hyperalgesia. Peridural fibrosis did not show any correlation with pain behaviors. There have been limited studies on the correlation between peridural fibrosis and pain behavioral change, which should be verified by further studies.
MeSH Terms
Figure
Reference
-
1. Gill GG, Sakovich L, Thompson E. Pedicle fat grafts for the prevention of scar formation after laminectomy. An experimental study in dogs. Spine (Phila Pa 1976). 1979; 4:176–186. PMID: 264034.
Article2. Kitano T, Zerwekh JE, Edwards ML, Usui Y, Allen MD. Viscous carboxymethylcellulose in the prevention of epidural scar formation. Spine (Phila Pa 1976). 1991; 16:820–823. PMID: 1925760.
Article3. Alkalay RN, Kim DH, Urry DW, Xu J, Parker TM, Glazer PA. Prevention of postlaminectomy epidural fibrosis using bioelastic materials. Spine (Phila Pa 1976). 2003; 28:1659–1665. PMID: 12897488.
Article4. Jacobs RR, McClain O, Neff J. Control of postlaminectomy scar formation: an experimental and clinical study. Spine (Phila Pa 1976). 1980; 5:223–229. PMID: 7394661.5. Kuivila TE, Berry JL, Bell GR, Steffee AD. Heparinized materials for control of the formation of the laminectomy membrane in experimental laminectomies in dogs. Clin Orthop Relat Res. 1988; 236:166–174. PMID: 3052976.
Article6. Einhaus SL, Robertson JT, Dohan FC Jr, Wujek JR, Ahmad S. Reduction of peridural fibrosis after lumbar laminotomy and discectomy in dogs by a resorbable gel (ADCON-L). Spine (Phila Pa 1976). 1997; 22:1440–1446. PMID: 9231961.
Article7. de Tribolet N, Porchet F, Lutz TW, Gratzl O, Brotchi J, van Alphen HA, et al. Clinical assessment of a novel antiadhesion barrier gel: prospective, randomized, multicenter, clinical trial of ADCON-L to inhibit postoperative peridural fibrosis and related symptoms after lumbar discectomy. Am J Orthop (Belle Mead NJ). 1998; 27:111–120. PMID: 9506196.8. Yong-Hing K, Reilly J, de Korompay V, Kirkaldy-Willis WH. Prevention of nerve root adhesions after laminectomy. Spine (Phila Pa 1976). 1980; 5:59–64. PMID: 7361199.
Article9. Mikawa Y, Hamagami H, Shikata J, Higashi S, Yamamuro T, Hyon SH, et al. An experimental study on prevention of postlaminectomy scar formation by the use of new materials. Spine (Phila Pa 1976). 1986; 11:843–846. PMID: 3643651.
Article10. Songer MN, Ghosh L, Spencer DL. Effects of sodium hyaluronate on peridural fibrosis after lumbar laminotomy and discectomy. Spine (Phila Pa 1976). 1990; 15:550–554. PMID: 2402695.
Article11. Henderson R, Weir B, Davis L, Mielke B, Grace M. Attempted experimental modification of the postlaminectomy membrane by local instillation of recombinant tissue-plasminogen activator gel. Spine (Phila Pa 1976). 1993; 18:1268–1272. PMID: 8211357.
Article12. Abitbol JJ, Lincoln TL, Lind BI, Amiel D, Akeson WH, Garfin SR. Preventing postlaminectomy adhesion. A new experimental model. Spine (Phila Pa 1976). 1994; 19:1809–1814. PMID: 7973979.13. Songer MN, Rauschning W, Carson EW, Pandit SM. Analysis of peridural scar formation and its prevention after lumbar laminotomy and discectomy in dogs. Spine (Phila Pa 1976). 1995; 20:571–580. PMID: 7604327.
Article14. Welch WC, Thomas KA, Cornwall GB, Gerszten PC, Toth JM, Nemoto EM, et al. Use of polylactide resorbable film as an adhesion barrier. J Neurosurg. 2002; 97(4 Suppl):413–422. PMID: 12449195.
Article15. Kato T, Haro H, Komori H, Shinomiya K. Evaluation of hyaluronic acid sheet for the prevention of postlaminectomy adhesions. Spine J. 2005; 5:479–488. PMID: 16153573.
Article16. Sunblad L. Studies on hyaluronic acid in synovial fluids. Acta Soc Med Ups. 1953; 58:113–238. PMID: 13065152.17. Rydell N, Balazs EA. Effect of intra-articular injection of hyaluronic acid on the clinical symptoms of osteoarthritis and on granulation tissue formation. Clin Orthop Relat Res. 1971; 80:25–32. PMID: 5002457.
Article18. Dixon AS, Jacoby RK, Berry H, Hamilton EB. Clinical trial of intra-articular injection of sodium hyaluronate in patients with osteoarthritis of the knee. Curr Med Res Opin. 1988; 11:205–213. PMID: 3063436.
Article19. Swann DA, Radin EL, Nazimiec M, Weisser PA, Curran N, Lewinnek G. Role of hyaluronic acid in joint lubrication. Ann Rheum Dis. 1974; 33:318–326. PMID: 4415649.
Article20. Tobetto K, Nakai K, Akatsuka M, Yasui T, Ando T, Hirano S. Inhibitory effects of hyaluronan on neutrophil-mediated cartilage degradation. Connect Tissue Res. 1993; 29:181–190. PMID: 8222645.
Article21. Sakakibara Y, Miura T, Iwata H, Kikuchi T, Yamaguchi T, Yoshimi T, et al. Effect of high-molecular-weight sodium hyaluronate on immobilized rabbit knee. Clin Orthop Relat Res. 1994; 299:282–292. PMID: 8119031.
Article22. Wobig M, Bach G, Beks P, Dickhut A, Runzheimer J, Schwieger G, et al. The role of elastoviscosity in the efficacy of viscosupplementation for osteoarthritis of the knee: a comparison of hylan G-F 20 and a lower-molecular-weight hyaluronan. Clin Ther. 1999; 21:1549–1562. PMID: 10509850.
Article23. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63. PMID: 7990513.
Article24. Massie JB, Schimizzi AL, Huang B, Kim CW, Garfin SR, Akeson WH. Topical high molecular weight hyaluronan reduces radicular pain post laminectomy in a rat model. Spine J. 2005; 5:494–502. PMID: 16153575.
Article25. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988; 32:77–88. PMID: 3340425.
Article26. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980; 20:441–462. PMID: 7387124.
Article27. Yamagami T, Matsui H, Tsuji H, Ichimura K, Sano A. Effects of laminectomy and retained extradural foreign body on cauda equina adhesion. Spine (Phila Pa 1976). 1993; 18:1774–1781. PMID: 8235860.
Article28. Lu X, Richardson PM. Responses of macrophages in rat dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1993; 22:334–341. PMID: 8315414.
Article29. Gotoh S, Onaya J, Abe M, Miyazaki K, Hamai A, Horie K, et al. Effects of the molecular weight of hyaluronic acid and its action mechanisms on experimental joint pain in rats. Ann Rheum Dis. 1993; 52:817–822. PMID: 8250613.
Article30. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992; 50:355–363. PMID: 1333581.
Article31. Takenobu Y, Katsube N, Marsala M, Kondo K. Model of neuropathic intermittent claudication in the rat: methodology and application. J Neurosci Methods. 2001; 104:191–198. PMID: 11164245.
Article32. Ririe DG, Vernon TL, Tobin JR, Eisenach JC. Age-dependent responses to thermal hyperalgesia and mechanical allodynia in a rat model of acute postoperative pain. Anesthesiology. 2003; 99:443–448. PMID: 12883418.
Article33. Rydell N. Decreased granulation tissue reaction after installment of hyaluronic acid. Acta Orthop Scand. 1970; 41:307–311. PMID: 4991991.
Article34. Weigel PH, Fuller GM, LeBoeuf RD. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J Theor Biol. 1986; 119:219–234. PMID: 3736072.
Article35. Balazs EA, Darzynkiewicz Z. Kulonen E, Pikkarainen J, editors. The effect of hyaluronic acid on fibroblasts, mononuclear phagocytes and lymphocytes. Biology of the fibroblast. 1973. London: Academic Press;p. 237–352.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention of Adhesion Syndrome after Extraocular Muscle Surgery in Rabbit
- Nonalcoholic Fatty Liver Disease: Lifestyle Modification
- Comparative Study for Preventive Effects of Intra-Abdominal Adhesion Using Cyclo-Oxygenase-2 Enzyme (COX-2) Inhibitor, Low Molecular Weight Heparin (LMWH), and Synthetic Barrier
- Chitosan Pad, Cellulose Membrane, or Gelatin Sponge for Peridural Bleeding: An Efficacy Study on a Lumbar Laminectomized Rat Model
- Histologic Changes of Vocal Fold Aging in a Rat Model