Korean J Physiol Pharmacol.
2010 Dec;14(6):407-412. 10.4196/kjpp.2010.14.6.407.
Induction of the Intrinsic Apoptotic Pathway by 3-Deazaadenosine Is Mediated by BAX Activation in HL-60 Cells
- Affiliations
-
- 1Department of Biochemistry, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea. hoshik@catholic.ac.kr
- 2Department of Surgery, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- 3Department of Anatomy, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- KMID: 2071713
- DOI: http://doi.org/10.4196/kjpp.2010.14.6.407
Abstract
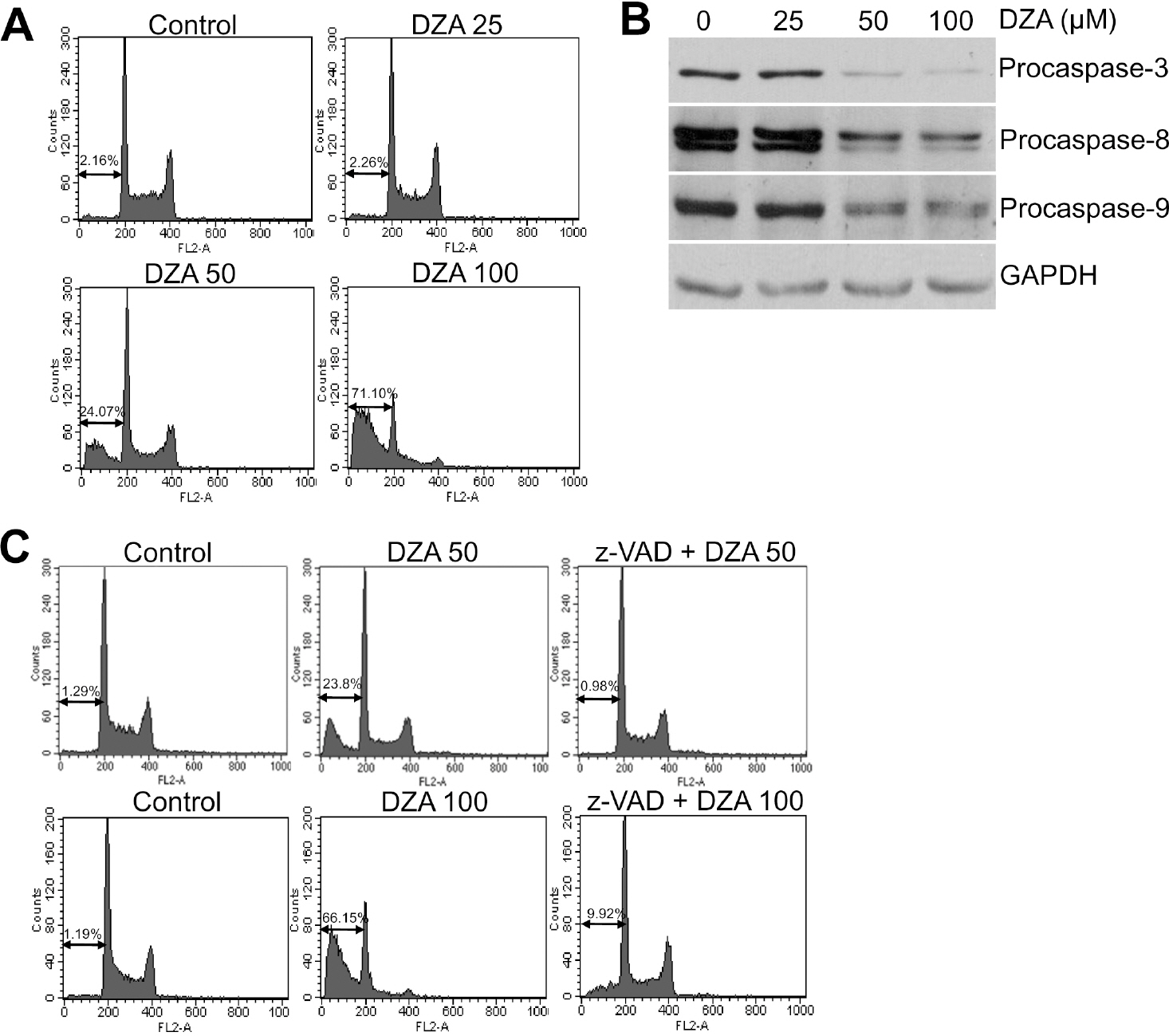
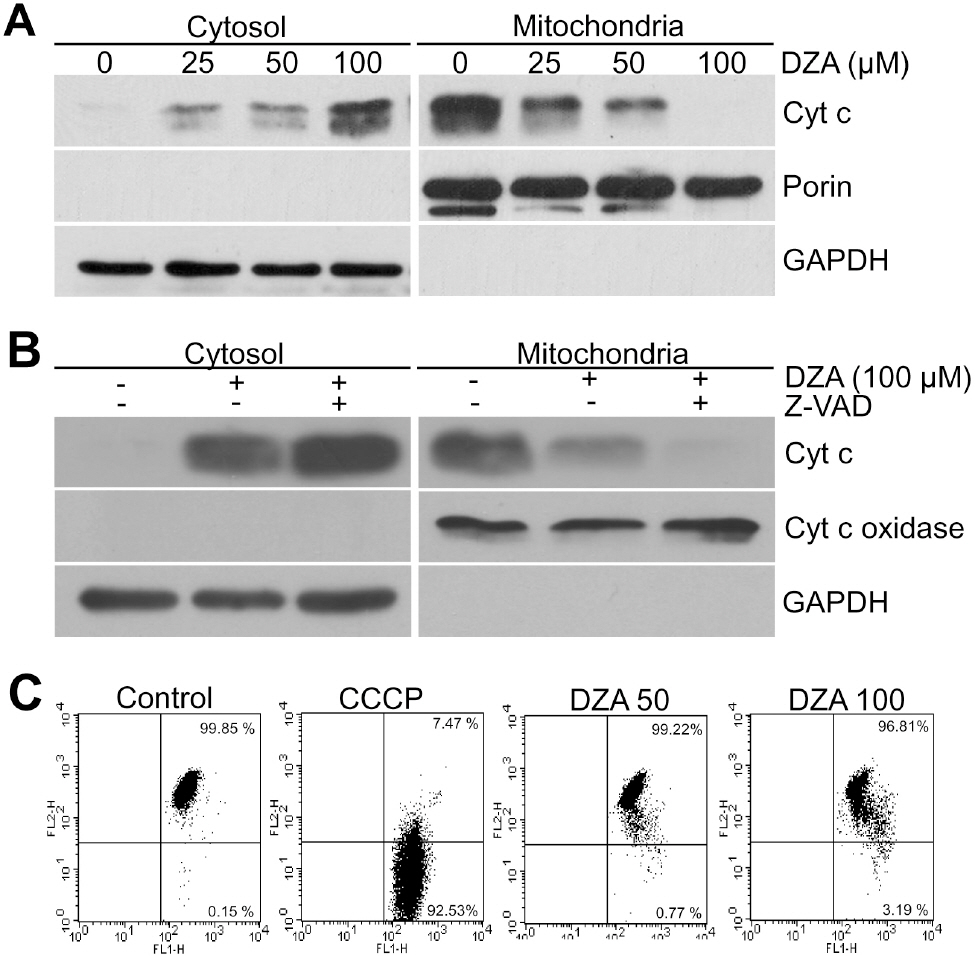
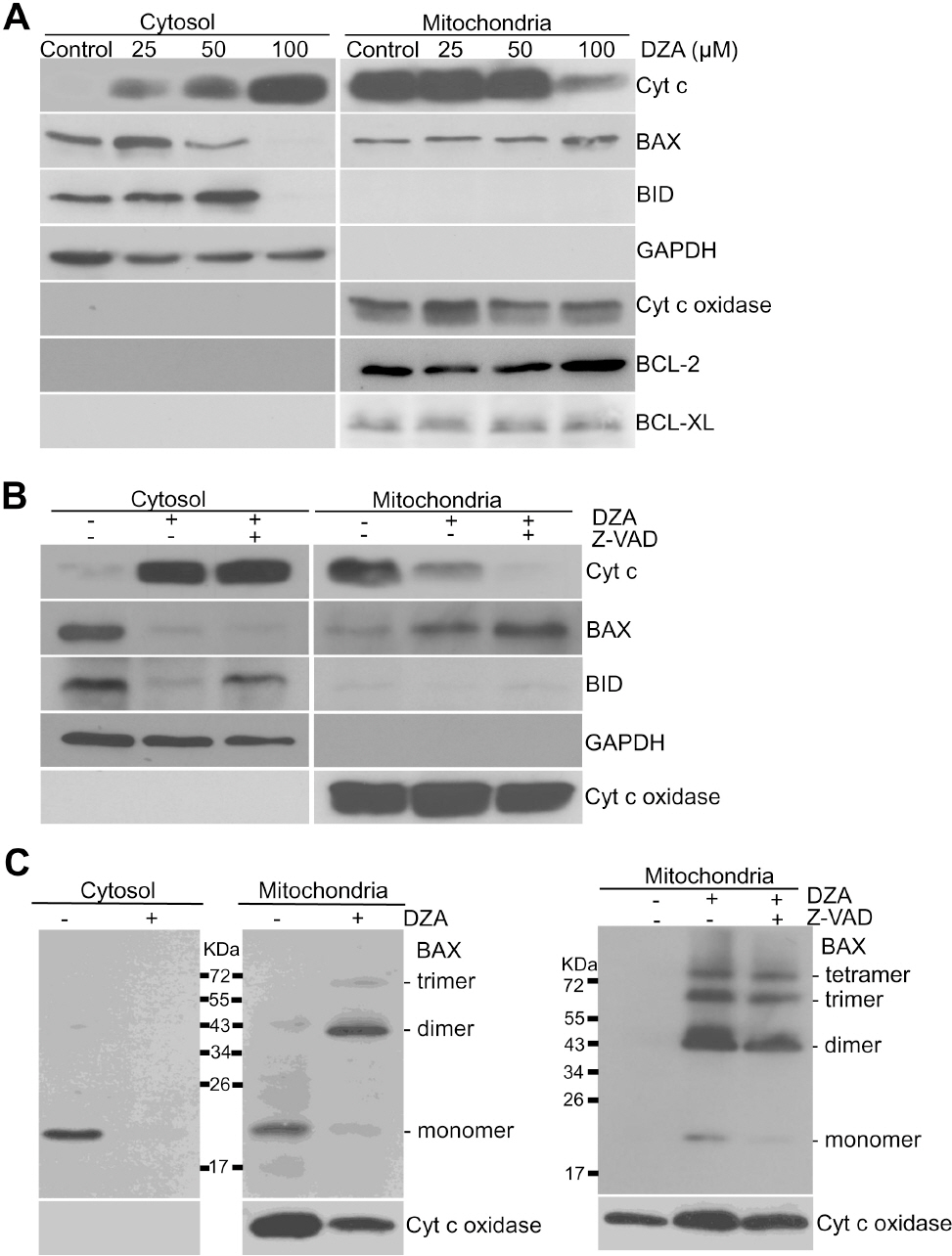
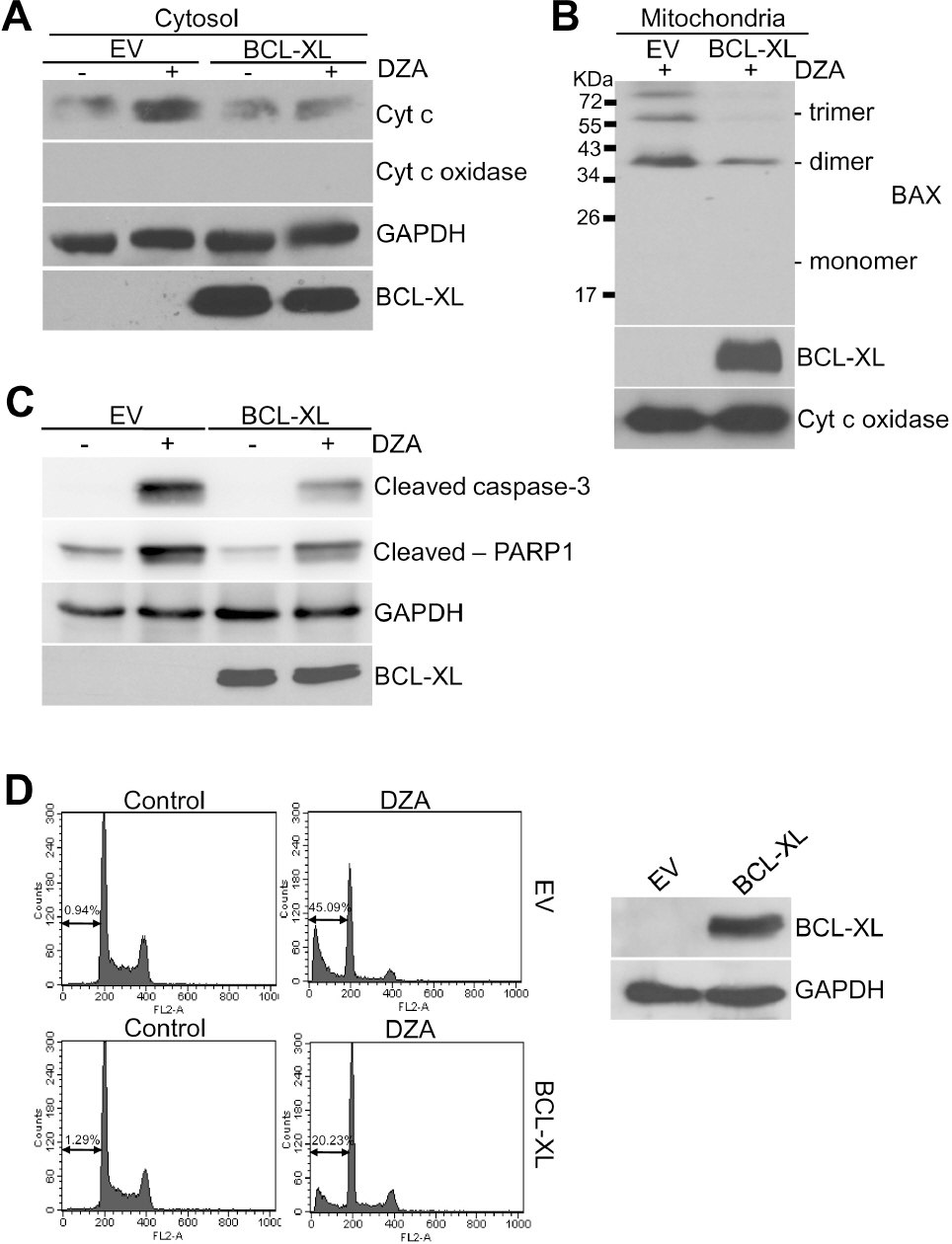
- 3-Deazaadenosine (DZA), a potent inhibitor of S-adenosylhomocysteine hydrolase, was previously proposed to induce intrinsic apoptosis in human leukemic cells. In the present study, we analyzed the mechanism underlying the DZA-induced intrinsic apoptotic pathway. DZA activated typical caspase-dependent apoptosis in HL-60 cells, as demonstrated by an accumulation of hypo-diploidic cells, the processing of multiple procaspases and an inhibitory effect of z-VAD-Fmk on this cell death. During DZA-induced apoptosis, cytochrome c (cyt c) was released into the cytosol. This was neither prevented by z-VAD-Fmk and nor was it associated with the dissipation of mitochondrial membrane potential (DeltaPsim). Prior to the release of cyt c, BAX was translocated from the cytosol to mitochondria and underwent oligomerization. Finally, the overexpression of BCL-XL protected HL-60 cells from apoptosis by blocking both the cyt c release and BAX oligomerization. Collectively, these findings suggest that DZA may activate intrinsic apoptosis by stimulating BAX activation and thereby the release of cyt c.
Keyword
MeSH Terms
-
Adenosylhomocysteinase
Amino Acid Chloromethyl Ketones
Apoptosis
bcl-2-Associated X Protein
bcl-X Protein
Cell Death
Cytochromes c
Cytosol
HL-60 Cells
Humans
Membrane Potential, Mitochondrial
Mitochondria
Tubercidin
Adenosylhomocysteinase
Amino Acid Chloromethyl Ketones
Cytochromes c
Tubercidin
bcl-2-Associated X Protein
bcl-X Protein
Figure
Reference
-
References
1. Chiang PK, Richards HH, Cantoni GL. S-Adenosyl-L-homocysteine hydrolase: analogues of S-adenosyl-L-homocysteine as potential inhibitors. Mol Pharmacol. 1977; 13:939–947.2. Aksamit RR, Falk W, Cantoni GL. Inhibition of chemotaxis by S-3-deazaadenosylhomocysteine in a mouse macrophage cell line. J Biol Chem. 1982; 257:621–625.
Article3. Chiang PK, Cantoni GL. Perturbation of biochemical transmethylations by 3-deazaadenosine in vivo. Biochem Pharmacol. 1979; 28:1897–1902.
Article4. Mayers DL, Mikovits JA, Joshi B, Hewlett IK, Estrada JS, Wolfe AD, Garcia GE, Doctor BP, Burke DS, Gordon RK, Lane JR, Chiang PK. Anti-human immunodeficiency virus 1 (HIV-1) activities of 3-deazaadenosine analogs: increased potency against-3′-azido-3′-deoxythymidine-resistant HIV-1 strains. Proc Natl Acad Sci USA. 1995; 92:215–219.5. Krenitsky TA, Rideout JL, Chao EY, Koszalka GW, Gurney F, Crouch RC, Cohn NK, Wolberg G, Vinegar R. Imidazo[4,5-c] pyridines (3-deazapurines) and their nucleosides as immunosuppressive and antiinflammatory agents. J Med Chem. 1986; 29:138–143.6. Chiang PK, Burbelo PD, Brugh SA, Gordon RK, Fukuda K, Yamada Y. Activation of collagen IV gene expression in F9 teratocarcinoma cells by 3-deazaadenosine analogs. Indirect inhibitors of methylation. J Biol Chem. 1992; 267:4988–4991.
Article7. Jurgensen CH, Huber BE, Zimmerman TP, Wolberg G. 3-deazaadenosine inhibits leukocyte adhesion and ICAM-1 biosynthesis in tumor necrosis factor-stimulated human endothelial cells. J Immunol. 1990; 144:653–661.8. Jeong SY, Lee JH, Kim HS, Hong SH, Cheong CH, Kim IK. 3-Deazaadenosine analogues inhibit the production of tumour necrosis factor-α in RAW264.7 cells stimulated with lipopolysaccharide. Immunology. 1996; 89:558–562.9. Jeong SY, Ahn SG, Lee JH, Kim HS, Kim JW, Rhim H, Jeong SW, Kim IK. 3-deazaadenosine, a S-adenosylhomocysteine hydrolase inhibitor, has dual effects on NF-kappaB regulation. Inhibition of NF-kappaB transcriptional activity and promotion of IκBα degradation. J Biol Chem. 1999; 274:18981–18988.10. Endresen PC, Eide TJ, Aarbakke J. Cell death initiated by 3-deazaadenosine in HL-60 cells is apoptosis and is partially inhibited by homocysteine. Biochem Pharmacol. 1993; 46:1893–1901.
Article11. Kim HS, Jeong SY, Lee JH, Kim BE, Kim JW, Jeong SW, Kim IK. Induction of apoptosis in human leukemia cells by 3-deazaadenosine is mediated by caspase-3-like activity. Exp Mol Med. 2000; 32:197–203.
Article12. Kim IK, Li CC, Young HA, Lee JH, Kim HS, Pardhasaradhi K, Garcia GE, Chiang PK. Apoptosis of L1210 Leukemia Cells Induced by 3-Deazaadenosine Analogs: Differential Expression of c-myc, NF-κB and Molecular Events. J Biomed Sci. 1997; 4:83–90.13. Chae YJ, Kim HS, Rhim H, Kim BE, Jeong SW, Kim IK. Activation of caspase-8 in 3-deazaadenosine-induced apoptosis of U-937 cells occurs downstream of caspase-3 and caspase-9 without Fas receptor-ligand interaction. Exp Mol Med. 2001; 33:284–292.
Article14. Byun HS, Won M, Park KA, Kim YR, Choi BL, Lee H, Hong JH, Piao L, Park J, Kim JM, Kweon GR, Kang SH, Han J, Hur GM. Prevention of TNF-induced necrotic cell death by rottlerin through a Nox1 NADPH oxidase. Exp Mol Med. 2008; 40:186–195.
Article15. Kim HJ, Song JY, Park HJ, Park HK, Yun DH, Chung JH. Narigin protects against rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Korean J Physiol Pharmacol. 2009; 13:281–285.16. Lee SY, Ahn JH, Ko KW, Kim J, Jeong SW, Kim IK, Kim J, Kim HS. Prostaglandin A2 activates intrinsic apoptotic pathway by direct interaction with mitochondria in HL-60 cells. Prostaglandins Other Lipid Mediat. 2010; 91:30–37.
Article17. Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, Green DR. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci USA. 2005; 102:17975–17980.
Article18. Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W, Duvvuri S, Taegtmeyer H, Andreeff M. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest. 2010; 120:142–156.
Article19. Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004; 305:626–629.
Article20. Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010; 11:621–632.
Article21. van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006; 16:203–213.
Article22. Khaled AR, Reynolds DA, Young HA, Thompson CB, Muegge K, Durum SK. Interleukin-3 withdrawal induces an early increase in mitochondrial membrane potential unrelated to the Bcl-2 family. Roles of intracellular pH, ADP transport, and F(0)F(1)-ATPase. J Biol Chem. 2001; 276:6453–6462.23. Li TW, Zhang Q, Oh P, Xia M, Chen H, Bemanian S, Lastra N, Circ M, Moyer MP, Mato JM, Aw TY, Lu SC. S-Adenosylmethionine and methylthioadenosine inhibit cellular FLICE inhibitory protein expression and induce apoptosis in colon cancer cells. Mol Pharmacol. 2009; 76:192–200.
Article24. Huang RF, Huang SM, Lin BS, Wei JS, Liu TZ. Homocysteine thiolactone induces apoptotic DNA damage mediated by increased intracellular hydrogen peroxide and caspase 3 activation in HL-60 cells. Life Sci. 2001; 68:2799–2811.
Article25. Cho YW, Jung HJ, Kim YK, Woo JS. A1 receptor-mediated protection against amyloid beta-induced injury in human neuroglioma cells. Korean J Physiol Pharmacol. 2007; 11:37–43.26. Zhenchuk A, Lotfi K, Juliusson G, Albertioni F. Mechanisms of anti-cancer action and pharmacology of clofarabine. Biochem Pharmacol. 2009; 78:1351–1359.
Article27. Parker WB, Shaddix SC, Chang CH, White EL, Rose LM, Brockman RW, Shortnacy AT, Montgomery JA, Secrist JA 3rd, Bennett LL Jr. Incorporation of the carbocyclic analog of 2′-deoxyguanosine into the DNA of herpes simplex virus and of HEp-2 cells infected with herpes simplex virus. Cancer Res. 1991; 51:2386–2394.28. Genini D, Budihardjo I, Plunkett W, Wang X, Carrera CJ, Cottam HB, Carson DA, Leoni LM. Nucleotide requirements for the in vitro activation of the apoptosis protein-activating factor-1-mediated caspase pathway. J Biol Chem. 2000; 275:29–34.
Article29. Prus KL, Wolberg G, Keller PM, Fyfe JA, Stopford CR, Zimmerman TP. 3-Deazaadenosine 5′-triphosphate: a novel metabolite of 3-deazaadenosine in mouse leukocytes. Biochem Pharmacol. 1989; 38:509–517.
Article30. Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008; 68:3421–3428.
Article31. Marzo I, Pérez-Galán P, Giraldo P, Rubio-Félix D, Anel A, Naval J. Cladribine induces apoptosis in human leukaemia cells by caspase-dependent and -independent pathways acting on mitochondria. Biochem J. 2001; 359:537–546.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on Apoptotic Signaling Pathway in HL-60 Cells Induced by Radiation
- Effects of cimifugin on cell growth inhibition and cell apoptosis induction in fadu human pharyngeal squamous cell carcinoma
- Induction of apoptosis in human leukemia cells by 3-deazaadenosine is mediated by caspase-3-like activity
- Inhibition of cell growth and induction of apoptosis by bilobalide in FaDu human pharyngeal squamous cell carcinoma
- Anti-tumor effect of new compound, 127, through the induction of apoptosis