J Korean Med Assoc.
2014 Apr;57(4):334-340. 10.5124/jkma.2014.57.4.334.
Aging and impaired hematopoiesis
- Affiliations
-
- 1Department of Laboratory Medicine, Medical School, Chonnam National University, Gwangju, Korea. mgshin@chonnam.ac.kr
- 2Center for Creative Biomedical Scientists, Chonnam National University, Gwangju, Korea.
- KMID: 2064891
- DOI: http://doi.org/10.5124/jkma.2014.57.4.334
Abstract
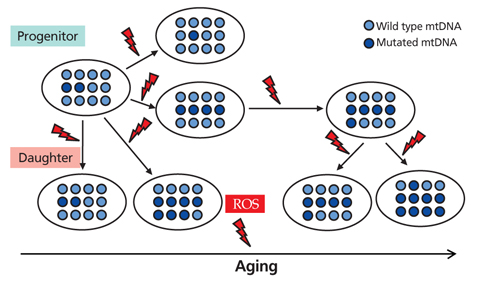
- Aging is an universal phenomenon and irreversible syndrome, and its damage occurs to molecules (DNA, proteins, and lipids), to cells, and to organs. Hematopoietic tissue intrinsically has a very high turnover rate; nonetheless, it is not protected from age-related insults. Aging results in the overproduction of myeloid cells, which leads to a pro-inflammatory environment. The selective expansion of a clonal subtype of intrinsically myeloid-biased hematopoietic stem cells (HSCs) is a central component of hematopoietic aging. In the present study, the stress-response and inflammatory genes were up-regulated with age whereas chromatin remodeling and DNA repair genes were down-regulated. Accumulated DNA damage, loss of DNA repair, and epigenetic deregulation are the main molecular mechanisms underlying age-dependent HSC decline. The most profound effect is seen in the adaptive immune system with a marked decline of lymphoid function in the elderly. Mitochondrial dysfunction and mitochondrial DNA mutation are another important contributor to the aging of HSCs, which have been regarded as a part of the mitochondrial theory of aging. Generation of reactive oxygen species during mitochondrial adenosine triphosphate generation, results in damage to mitochondria and mitochondrial DNA, the latter leading to deleterious mutations that directly caused the functional decline of human. Studies have pointed toward intrinsic deficits in HSC function, and epigenetic deregulation as the important contributing factors behind hematopoietic decline and malignancy during aging. Aging-related changes such as hematopoiesis are reflected by a decline in marrow cellularity, increased risk of anemia, marrow failure syndrome, and myeloproliferative neoplasms as well as a decline of adaptive immunity.
Keyword
MeSH Terms
-
Adaptive Immunity
Adenosine Triphosphate
Aged
Aging*
Anemia
Bone Marrow
Chromatin Assembly and Disassembly
DNA Damage
DNA Repair
DNA, Mitochondrial
Epigenomics
Hematopoiesis*
Hematopoietic Stem Cells
Humans
Immune System
Mitochondria
Myeloid Cells
Reactive Oxygen Species
Adenosine Triphosphate
DNA, Mitochondrial
Reactive Oxygen Species
Figure
Reference
-
1. Beghe C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004; 116:3S–10S.
Article2. Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007; 70:179–189.
Article3. Lichtman MA, Rowe JM. The relationship of patient age to the pathobiology of the clonal myeloid diseases. Semin Oncol. 2004; 31:185–197.
Article4. Bowen RL, Atwood CS. Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontology. 2004; 50:265–290.5. Ergen AV, Goodell MA. Mechanisms of hematopoietic stem cell aging. Exp Gerontol. 2010; 45:286–290.
Article6. Haynes BF, Sempowski GD, Wells AF, Hale LP. The human thymus during aging. Immunol Res. 2000; 22:253–261.
Article7. Edwards BK, Howe HL, Ries LA, Thun MJ, Rosenberg HM, Yancik R, Wingo PA, Jemal A, Feigal EG. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002; 94:2766–2792.
Article8. Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008; 132:681–696.
Article9. Beerman I, Maloney WJ, Weissmann IL, Rossi DJ. Stem cells and the aging hematopoietic system. Curr Opin Immunol. 2010; 22:500–506.
Article10. Snoeck HW. Aging of the hematopoietic system. Curr Opin Hematol. 2013; 20:355–361.
Article11. Charbord P, Tavian M, Humeau L, Peault B. Early ontogeny of the human marrow from long bones: an immunohistochemical study of hematopoiesis and its microenvironment. Blood. 1996; 87:4109–4119.
Article12. Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005; 106:1479–1487.
Article13. Leng SX, Hung W, Cappola AR, Yu Q, Xue QL, Fried LP. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2009; 64:499–502.
Article14. Patel KV, Harris TB, Faulhaber M, Angleman SB, Connelly S, Bauer DC, Kuller LH, Newman AB, Guralnik JM. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007; 109:4663–4670.
Article15. Chaves PH, Xue QL, Guralnik JM, Ferrucci L, Volpato S, Fried LP. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? J Am Geriatr Soc. 2004; 52:1811–1816.
Article16. Rao KM, Currie MS, Padmanabhan J, Cohen HJ. Age-related alterations in actin cytoskeleton and receptor expression in human leukocytes. J Gerontol. 1992; 47:B37–B44.
Article17. Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005; 205:257–268.
Article18. Nilsson-Ehle H, Jagenburg R, Landahl S, Svanborg A, Westin J. Haematological abnormalities and reference intervals in the elderly. A cross-sectional comparative study of three urban Swedish population samples aged 70, 75 and 81 years. Acta Med Scand. 1988; 224:595–604.19. Taraldsrud E, Grogaard HK, Solheim S, Lunde K, Floisand Y, Arnesen H, Seljeflot I, Egeland T. Age and stress related phenotypical changes in bone marrow CD34+ cells. Scand J Clin Lab Invest. 2009; 69:79–84.20. Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weiss-man IL, Bryder D, Rossi DJ. Functionally distinct hema-topoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010; 107:5465–5470.
Article21. Rossi DJ, Bryder D, Weissman IL. Hematopoietic stem cell aging: mechanism and consequence. Exp Gerontol. 2007; 42:385–390.
Article22. Kamminga LM, Bystrykh LV, de Boer A, Houwer S, Douma J, Weersing E, Dontje B, de Haan G. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood. 2006; 107:2170–2179.
Article23. Shin MG, Kajigaya S, McCoy JP Jr, Levin BC, Young NS. Marked mitochondrial DNA sequence heterogeneity in single CD34+ cell clones from normal adult bone marrow. Blood. 2004; 103:553–561.
Article24. Shin MG, Kajigaya S, Tarnowka M, McCoy JP Jr, Levin BC, Young NS. Mitochondrial DNA sequence heterogeneity in circulating normal human CD34 cells and granulocytes. Blood. 2004; 103:4466–4477.
Article25. Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003; 425:836–841.
Article26. Mari D, Coppola R, Provenzano R. Hemostasis factors and aging. Exp Gerontol. 2008; 43:66–73.
Article27. Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007; 211:144–156.
Article