J Korean Med Assoc.
2013 Sep;56(9):762-770. 10.5124/jkma.2013.56.9.762.
Neurobiology, pharmacokinetics and pharmacodynamics of drug abuse
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Yonsei University College of Medicine, Seoul, Korea. hanesth@yuhs.ac.kr
- 2Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2015672
- DOI: http://doi.org/10.5124/jkma.2013.56.9.762
Abstract
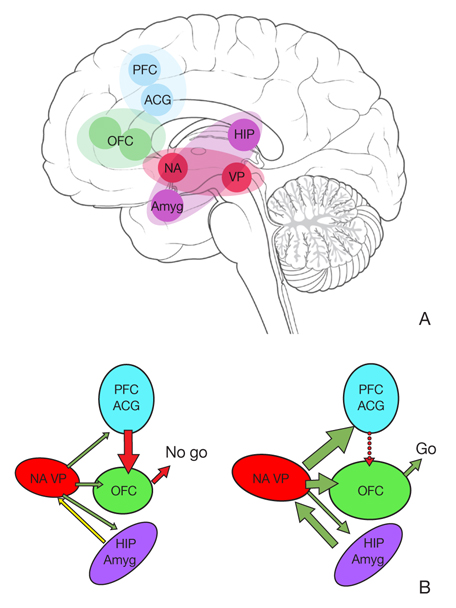
- All drugs of abuse, like neural rewarding behaviors such as sex and eating, increase extra-cellular dopamine (DA) levels in the nucleus accumbens (NA), which is a part of the common reward mesolimbic pathway from the ventral tegmental area (VTA) to the NA. As addiction progresses from initial use to obsessive compulsive use, the neurobiology shifts from a DA-based behavioral system to a predominantly glutamate-based one, still relying on DA. A DA release in the prefrontal cortex (PFC) and amygdala in the relapse stimulates glutamate transmission between the PFC and amygdala and glutamate release in the pathway from the PFC to the NA core, constituting a "final common pathway" for drug-seeking behavior. Dysfunction of critical PFC structures results in drug craving and impaired decision making. Inhalation and smoking are the routes of administration that allow the most rapid delivery of drugs to the brain, while intravenous injection maximizes the bioavailability of a drug. The pharmacokinetic properties of a drug that dispose the user to increased self-administration include rapid absorption, rapid entry into the central nervous system, high bioavailability, short half-life, small volume of distribution, and high free drug clearance. The pharmacokinetic properties associated with drug dependence are a long half-life, low free drug clearance, and presence of the drug at high enough concentrations and for a sufficient time to permit tolerance to develop. Pharmacokinetics and pharmacodynamics play an important role in predicting the dependence and abuse potential of drugs.
MeSH Terms
-
Absorption
Amygdala
Biological Availability
Brain
Central Nervous System
Decision Making
Dopamine
Drug-Seeking Behavior
Eating
Glutamic Acid
Half-Life
Inhalation
Injections, Intravenous
Neurobiology
Nucleus Accumbens
Prefrontal Cortex
Recurrence
Reward
Smoke
Smoking
Street Drugs
Substance-Related Disorders
Ventral Tegmental Area
Dopamine
Glutamic Acid
Smoke
Street Drugs
Figure
Reference
-
1. Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005; 162:1403–1413.
Article2. Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006; 12:559–566.
Article3. Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev. 1993; 18:75–113.
Article4. Heidbreder C. Novel pharmacotherapeutic targets for the management of drug addiction. Eur J Pharmacol. 2005; 526:101–112.
Article5. Wise RA. Action of drugs of abuse on brain reward systems. Pharmacol Biochem Behav. 1980; 13:Suppl 1. 213–223.
Article6. Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005; 162:1414–1422.
Article7. Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse: neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001; 937:1–26.8. Kalivas PW. Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialogues Clin Neurosci. 2007; 9:389–397.
Article9. Ross S, Peselow E. The neurobiology of addictive disorders. Clin Neuropharmacol. 2009; 32:269–276.
Article10. Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004; 24:11017–11022.
Article11. Busto U, Sellers EM. Pharmacokinetic determinants of drug abuse and dependence: a conceptual perspective. Clin Pharmacokinet. 1986; 11:144–153.12. Sakol MS, Stark C, Sykes R. Buprenorphine and temazepam abuse by drug takers in Glasgow: an increase. Br J Addict. 1989; 84:439–441.13. Jones RT. Psychopharmacology of cocaine. In : Washton AM, Gold MS, editors. Cocaine: a clinician's handbook. New York: Guildford Press;1987. p. 55–72.14. Brosen K. Recent developments in hepatic drug oxidation. Implications for clinical pharmacokinetics. Clin Pharmacokinet. 1990; 18:220–239.15. Quinn DI, Wodak A, Day RO. Pharmacokinetic and pharmacodynamic principles of illicit drug use and treatment of illicit drug users. Clin Pharmacokinet. 1997; 33:344–400.
Article16. Milne RW, Nation RL, Somogyi AA, Bochner F, Griggs WM. The influence of renal function on the renal clearance of morphine and its glucuronide metabolites in intensive-care patients. Br J Clin Pharmacol. 1992; 34:53–59.
Article17. Cook CE. Pyrolytic characteristics, pharmacokinetics, and bioavailability of smoked heroin, cocaine, phencyclidine, and methamphetamine. NIDA Res Monogr. 1991; 115:6–23.
Article18. Nilsson MI, Meresaar U, Anggard E. Clinical pharmacokinetics of methadone. Acta Anaesthesiol Scand Suppl. 1982; 74:66–69.
Article19. Harding-Pink D. Methadone: one person's maintenance dose is another's poison. Lancet. 1993; 341:665–666.20. Kleber HD, Kosten TR, Gaspari J, Topazian M. Nontolerance to the opioid antagonism of naltrexone. Biol Psychiatry. 1985; 20:66–72.
Article21. Bullingham RE, McQuay HJ, Porter EJ, Allen MC, Moore RA. Sublingual buprenorphine used postoperatively: ten hour plasma drug concentration analysis. Br J Clin Pharmacol. 1982; 13:665–673.
Article22. Fudala PJ, Jaffe JH, Dax EM, Johnson RE. Use of buprenorphine in the treatment of opioid addiction. II. Physiologic and behavioral effects of daily and alternate-day administration and abrupt withdrawal. Clin Pharmacol Ther. 1990; 47:525–534.
Article23. Jarrott B, Conway EL, Maccarrone C, Lewis SJ. Clonidine: understanding its disposition, sites and mechanism of action. Clin Exp Pharmacol Physiol. 1987; 14:471–479.
Article24. Washton AM, Resnick RB, Geyer G. Opiate withdrawal using lofexidine, a clonidine analogue with fewer side effects. J Clin Psychiatry. 1983; 44:335–337.25. Foltin RW, Fischman MW. Self-administration of cocaine by humans: choice between smoked and intravenous cocaine. J Pharmacol Exp Ther. 1992; 261:841–849.26. Morishima HO, Whittington RA. Species-, gender-, and pregnancy-related differences in the pharmacokinetics and pharmacodynamics of cocaine. NIDA Res Monogr. 1995; 158:2–21.
Article27. Anggård E, Gunne LM, Jönsson LE. Relationships between pharmacokinetic and clinical parameters in chronic amphetamine abuse. Acta Pharmacol Toxicol (Copenh). 1970; 28:92.28. Ricaurte GA, McCann UD. Neurotoxic amphetamine analogues: effects in monkeys and implications for humans. Ann N Y Acad Sci. 1992; 648:371–382.
Article29. Lindgren JE, Ohlsson A, Agurell S, Hollister L, Gillespie H. Clinical effects and plasma levels of delta 9-tetrahydrocannabinol (delta 9-THC) in heavy and light users of cannabis. Psychopharmacology (Berl). 1981; 74:208–212.
Article30. Griffiths RR, Ator NA, Lukas SE, Lamb RJ, Brady JV. Benzodiazepines: drug discrimination and physiological dependence. NIDA Res Monogr. 1984; 49:163–164.
Article31. Ballinger BR. New drugs. Hypnotics and anxiolytics. BMJ. 1990; 300:456–458.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacokinetics and pharmacodynamics of drugs for sedation
- Current Updates in Pharmacokinetics and Pharmacodynamics of Fluoroquinolones
- How to design intravenous anesthetic dose regimens based on pharmacokinetics and pharmacodynamics principles
- Pharmacokinetics and Pharmacodynamics of Antibiotics : General Concepts and Recent Advances
- Psychotropics Metabolism: Gender-Related Issues