Anat Cell Biol.
2012 Jun;45(2):121-127. 10.5115/acb.2012.45.2.121.
Purkinje cells loss in off spring due to maternal morphine sulfate exposure: a morphometric study
- Affiliations
-
- 1Gorgan Congenital Malformations Research Center, Department of Anatomical Sciences, Gorgan, Iran. mjgolalipour@yahoo.com
- 2Department of Anatomical Sciences, Faculty of Medicine, Golestan University of Medical Sciences, Gorgan, Iran.
- KMID: 2005875
- DOI: http://doi.org/10.5115/acb.2012.45.2.121
Abstract
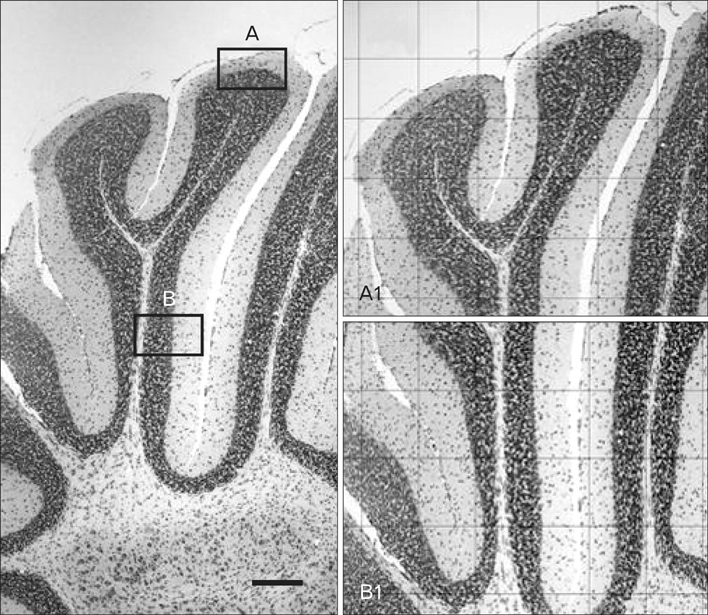
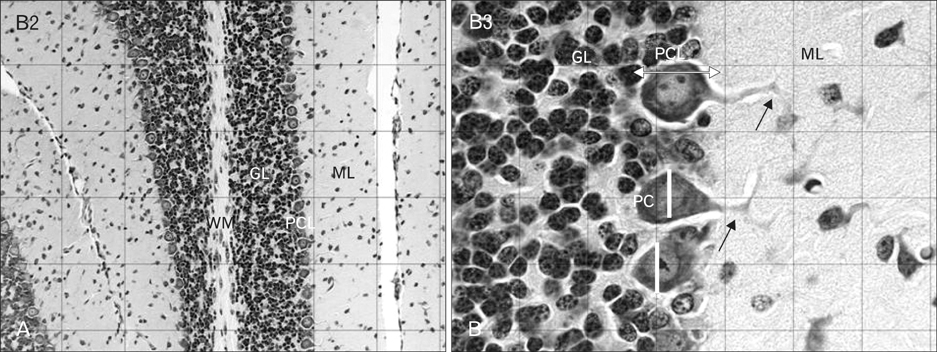
- The toxic effects of morphine sulfate in the adult cerebral cortex and one-day neonatal cerebellum have been studied. This study was carried out to evaluate the effect of maternal morphine exposure during gestational and lactation period on the Purkinje cells and cerebellar cortical layer in 18- and 32-day-old mice offspring. Thirty female mice were randomly allocated into cases and controls. In cases, animals received morphine sulfate (10 mg/kg/body weight intraperitoneally) during the 7 days before mating, gestational day (GD 0-21) 18 or 32. The controls received an equivalent volume of saline. The cerebellum of six infants for each group was removed and each was stained with cresyl violet. Quantitative computer-assisted morphometric study was done on cerebellar cortex. The linear Purkinje cell density in both experimental groups (postnatal day [P]18, 23.40+/-0.5; P32, 23.45+/-1.4) were significantly reduced in comparison with the control groups (P18, 28.70+/-0.9; P32, 28.95+/-0.4) (P<0.05). Purkinje cell area, perimeter and diameter at apex and depth of simple lobules in the experimental groups were significantly reduced compared to the controls (P<0.05). The thickness of the Purkinje layer of the cerebellar cortex was significantly reduced in morphine treated groups (P<0.05). This study reveals that morphine administration before pregnancy, during pregnancy and during the lactation period causes Purkinje cells loss and Purkinje cell size reduction in 18- and 32-day-old infant mice.
Keyword
MeSH Terms
Figure
Reference
-
1. Zhang Y, Chen Q, Yu LC. Morphine: a protective or destructive role in neurons? Neuroscientist. 2008. 14:561–570.2. National Institute on Drug Abuse. National pregnancy and health survey: drug use among women delivering livebirths, 1992 (NIH Publication 96-3819). 1996. Rockville, MD: U. S. Department of Health and Human Services, National Institutes of Health, National Institute of Drug Abuse, Division of Epidemiology and Prevention Research;1–157.3. Ornoy A, Michailevskaya V, Lukashov I, Bar-Hamburger R, Harel S. The developmental outcome of children born to heroindependent mothers, raised at home or adopted. Child Abuse Negl. 1996. 20:385–396.4. Raye JR, Dubin JW, Blechner JN. Fetal growth retardation following maternal morphine administration: nutritional or drug effect? Biol Neonate. 1977. 32:222–228.5. Wilson GS, McCreary R, Kean J, Baxter JC. The development of preschool children of heroin-addicted mothers: a controlled study. Pediatrics. 1979. 63:135–141.6. Fazel A, Jalali M. Experimentally-induced exencephaly and spina bifida in mice. Arch Iran Med. 2002. 5:179–183.7. Nasiraei-Moghadam S, Sahraei H, Bahadoran H, Sadooghi M, Salimi SH, Kaka GR, Imani H, Mahdavi-Nasab H, Dashtnavard H. Effects of maternal oral morphine consumption on neural tube development in Wistar rats. Brain Res Dev Brain Res. 2005. 159:12–17.8. Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002. 22:7650–7661.9. Atici S, Cinel L, Cinel I, Doruk N, Aktekin M, Akca A, Camdeviren H, Oral U. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Int J Neurosci. 2004. 114:1001–1011.10. Turchan-Cholewo J, Liu Y, Gartner S, Reid R, Jie C, Peng X, Chen KC, Chauhan A, Haughey N, Cutler R, Mattson MP, Pardo C, Conant K, Sacktor N, McArthur JC, Hauser KF, Gairola C, Nath A. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and L-deprenyl. Neurobiol Dis. 2006. 23:109–119.11. Sadraie SH, Kaka GR, Sahraei H, Dashtnavard H, Bahadoran H, Mofid M, Nasab HM, Jafari F. Effects of maternal oral administration of morphine sulfate on developing rat fetal cerebrum: a morphometrical evaluation. Brain Res. 2008. 1245:36–40.12. Bekheet SH, Saker SA, Abdel-Kader AM, Younis AE. Histopathological and biochemical changes of morphine sulphate administration on the cerebellum of albino rats. Tissue Cell. 2010. 42:165–175.13. Ghafari S, Roshandel D, Golalipour MJ. Effect of intrauterine morphine sulfate exposure on cerebellar histomorphological changes in neonatal mice. Folia Neuropathol. 2011. 49:328–334.14. Lee Y, Rowe J, Eskue K, West JR, Maier SE. Alcohol exposure on postnatal day 5 induces Purkinje cell loss and evidence of Purkinje cell degradation in lobule I of rat cerebellum. Alcohol. 2008. 42:295–302.15. Abou-Donia MB, Khan WA, Dechkovskaia AM, Goldstein LB, Bullman SL, Abdel-Rahman A. In utero exposure to nicotine and chlorpyrifos alone, and in combination produces persistent sensorimotor deficits and Purkinje neuron loss in the cerebellum of adult off spring rats. Arch Toxicol. 2006. 80:620–631.16. Hauser KF, Gurwell JA, Turbek CS. Morphine inhibits Purkinje cell survival and dendritic differentiation in organotypic cultures of the mouse cerebellum. Exp Neurol. 1994. 130:95–105.17. Hauser KF, Harris-White ME, Jackson JA, Opanashuk LA, Carney JM. Opioids disrupt Ca2+ homeostasis and induce carbonyl oxyradical production in mouse astrocytes in vitro: transient increases and adaptation to sustained exposure. Exp Neurol. 1998. 151:70–76.18. Hauser KF, Houdi AA, Turbek CS, Elde RP, Maxson W 3rd. Opioids intrinsically inhibit the genesis of mouse cerebellar granule neuron precursors in vitro: differential impact of mu and delta receptor activation on proliferation and neurite elongation. Eur J Neurosci. 2000. 12:1281–1293.19. Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioids regulate dendritic growth and spine formation in developing rat brain. Brain Res. 1987. 416:157–161.20. Lorber BA, Freitag SK, Bartolome JV. Effects of beta-endorphin on DNA synthesis in brain regions of preweanling rats. Brain Res. 1990. 531:329–332.21. Schmahl W, Funk R, Miaskowski U, Plendl J. Long-lasting effects of naltrexone, an opioid receptor antagonist, on cell proliferation in developing rat forebrain. Brain Res. 1989. 486:297–300.22. Hammer RP Jr, Ricalde AA, Seatriz JV. Effects of opiates on brain development. Neurotoxicology. 1989. 10:475–483.23. Zagon IS, McLaughlin PJ. Endogenous opioid systems regulate cell proliferation in the developing rat brain. Brain Res. 1987. 412:68–72.24. Oehmichen M, Meissner C, Reiter A, Birkholz M. Neuropathology in non-human immunodeficiency virus-infected drug addicts: hypoxic brain damage after chronic intravenous drug abuse. Acta Neuropathol. 1996. 91:642–646.25. Zagon IS, McLaughlin PJ. Identification of opioid peptides regulating proliferation of neurons and glia in the developing nervous system. Brain Res. 1991. 542:318–323.26. Hauser KF, Osborne JG, Stiene-Martin A, Melner MH. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Res. 1990. 522:347–353.27. Spruce BA, Curtis R, Wilkin GP, Glover DM. A neuropeptide precursor in cerebellum: proenkephalin exists in subpopulations of both neurons and astrocytes. EMBO J. 1990. 9:1787–1795.28. Shinoda H, Marini AM, Cosi C, Schwartz JP. Brain region and gene specificity of neuropeptide gene expression in cultured astrocytes. Science. 1989. 245:415–417.29. Zhu Y, Hsu MS, Pintar JE. Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. J Neurosci. 1998. 18:2538–2549.30. Goldstein A. Binding selectivity profiles for ligands of multiple receptor types: focus on opioid receptors. Trends Pharmacol Sci. 1987. 8:456–459.31. Slotkin TA. Perinatal exposure to methadone: how do early biochemical alterations cause neurofunctional disturbances? Prog Brain Res. 1988. 73:265–279.32. Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Brain Res Dev Brain Res. 1998. 105:159–166.33. Cheng WH, Quimby FW, Lei XG. Impacts of glutathione peroxidase-1 knockout on the protection by injected selenium against the pro-oxidant-induced liver aponecrosis and signaling in selenium-deficient mice. Free Radic Biol Med. 2003. 34:918–927.34. Pretorius E, Bornman MS. Calcium-mediated aponecrosis plays a central role in the pathogenesis of estrogenic chemical-induced neurotoxicity. Med Hypotheses. 2005. 65:893–904.35. van Tonder JJ. Effect of the cardiac glycoside, digoxin, on neuronal viability, serotonin production and brain development in the embryo. 2007. Pretoria: School of Medicine, Faculty of Health Science, University of Pretoria;MSc Thesis.36. Garcia MM, Gilster J, Harlan RE. Chronic morphine decreases calbindin D28k immunoreactivity in a subset of cerebellar Purkinje neurons of rat brain. Brain Res. 1996. 734:123–134.37. Kim JS, Kim JM, Son JA, Han SY, Kim CT, Lee NS, Jeong YG. Decreased calbindin-immunoreactive Renshaw cells (RCs) in the lumbar spinal cord of the ataxic pogo mice. Korean J Anat. 2008. 41:255–263.38. Farber NB, Olney JW. Drugs of abuse that cause developing neurons to commit suicide. Brain Res Dev Brain Res. 2003. 147:37–45.39. Darmanto W, Inouye M, Takagishi Y, Ogawa M, Mikoshiba K, Murata Y. Derangement of Purkinje cells in the rat cerebellum following prenatal exposure to X-irradiation: decreased Reelin level is a possible cause. J Neuropathol Exp Neurol. 2000. 59:251–262.40. Fam A. Depressant effects of morphine on cellular composition of lymphoid tissues and cerebellum in rats. J Egypt Ger Soc Zool. 2002. 39:411–438.41. Demeri O, Celik I, Seker M, Sur E, Ozkan Y, Aydin MF, Salbacak A. Histological and morphometric studies of the cerebellar cortex and nucleolus organiser region (Ag-NORs) in Purkinje neurons of chronic morphine treated rats. Proceedings of the XVII International Symposium on Morphological Science. 2002. Sep 11-15; Timisoara, Romania.42. Simat M, Parpan F, Fritschy JM. Heterogeneity of glycinergic and gabaergic interneurons in the granule cell layer of mouse cerebellum. J Comp Neurol. 2007. 500:71–83.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mediation of N-methyl-D-aspartate on neuropeptide Y expression induced by morphine in mouse cerebellum
- Spontaneous electrical activity in cerebellar Purkinje neurons of postnatal rats
- Laser Capture Microdissection Reveals Specific Genes Related to Purkinje Cell Death in the Leaner Mice
- Changes of Calbindin -D28k Expression Levels After Transient Ischemic Damage in Rat Cerebellar Purkinje Cells
- Abnormal Expression of Neuropeptide Y in the Cerebellar Purkinje Cells of the Ataxic Mutant Mice, Pogo