Korean J Urol.
2007 Apr;48(4):439-443. 10.4111/kju.2007.48.4.439.
Modulation of the Host Antimicrobial Peptide (Human beta-defensin-1, -2) Expression of Vaginal Squamous Epithelial Cells with using 17beta-Estradiol and Progesterone
- Affiliations
-
- 1Department of Urology, College of Medicine, Chung-Ang University, Seoul, Korea. uromyung@ yahoo.co.kr
- KMID: 1997113
- DOI: http://doi.org/10.4111/kju.2007.48.4.439
Abstract
- PURPOSE
In mammals, alphaand beta-defensins are antimicrobial peptides that are expressed in various epithelial and phagocytic cells. Human beta-defensin-1 and -2 (hBD-1, hBD-2) have recently been shown to be expressed in various epithelial cells. Vaginal mucosa can be a target of vaginitis and the site of uropathogens' colonization that precedes urinary tract infections. Therefore, innate host defense mediators like antimicrobial peptides in the vaginal mucosa are important. Estrogen and progesterone receptors have been shown to be expressed in the vaginal squamous epithelium. Sex hormones like estrogen and progesterone may cause vaginal atrophy or susceptibility to uropathogens. So, we performed this study to investigate the expression patterns of hBD-1 and -2 mRNA in vaginal squamous epithelium (VSE) with using lipopolysaccharide (LPS), 17beta-estradiol and progesterone.
MATERIALS AND METHODS
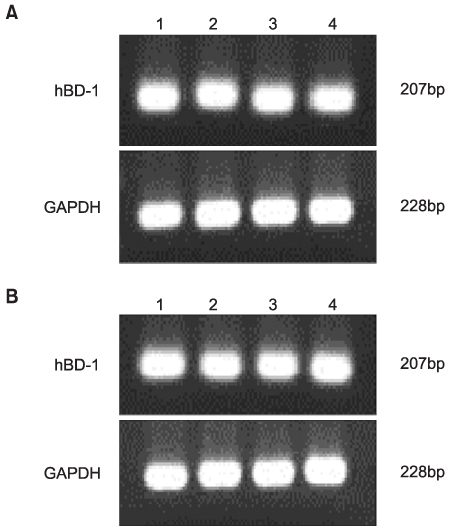
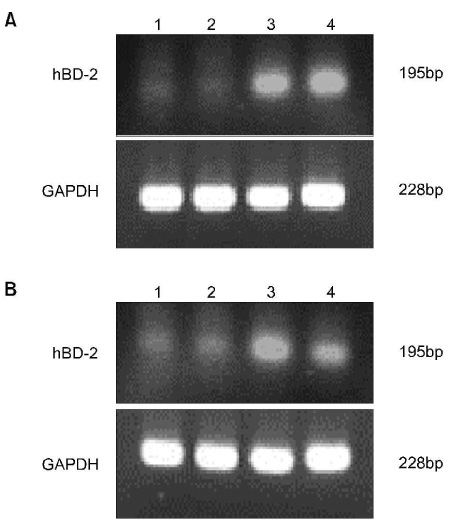
Normal VSE cells that were retrieved from vaginal tissue during vaginoplasty were primarily cultured in keratinocyte growth medium and they we allowed to undergo their 3rd passage. Modulation of the expressions of hBD-1 and -2 mRNA by various stimuli (LPS 0.5microgram/ml, E2 2nM, P 1micrometer) was measured by semiquantitative reverse transcription polymerase chain reaction (RT-PCR).
RESULTS
HBD-1 and -2 were constitutively expressed in the normal VSE cell lines, but the hBD-2 expression was not significant. A marked increase of the constitutive expression of hBD-2 mRNA was observed upon stimulation with LPS, but not upon stimulation with E2. A moderate decrease of the constitutive expression of hBD-2 mRNA upon stimulation with LPS was observed with administering progesterone.
CONCLUSIONS
These expressions of hBD-2 mRNA may have important roles in the innate host defense of the urogenital area. Artificial intake of progesterone may lead to susceptibility via a decrease of defensins.
Keyword
MeSH Terms
-
Atrophy
beta-Defensins
Cell Line
Colon
Defensins
Epithelial Cells*
Epithelium
Estrogens
Gonadal Steroid Hormones
Humans
Keratinocytes
Mammals
Mucous Membrane
Peptides
Phagocytes
Polymerase Chain Reaction
Progesterone*
Receptors, Progesterone
Reverse Transcription
RNA, Messenger
Urinary Tract Infections
Vagina
Vaginitis
Defensins
Estrogens
Gonadal Steroid Hormones
Peptides
Progesterone
RNA, Messenger
Receptors, Progesterone
beta-Defensins
Figure
Reference
-
1. Radtke AL, O'Riordan MX. Intracellular innate resistance to bacterial pathogens. Cell Microbiol. 2006. 8:1720–1729.2. Tan BH, Meinken C, Bastian M, Bruns H, Leqaspi A, Ochoa MT, et al. Marcrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol. 2006. 177:1864–1871.3. Schonwetter BS, Stolzenberg ED, Zasleff MA. Epithelial antibiotics induced at sites of inflammation. Science. 1995. 267:1645–1648.4. Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004. 75:34–38.5. Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997. 387:861.6. Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995. 368:331–335.7. Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB Jr, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998. 101:1633–1642.8. Schroder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999. 31:645–651.9. Abiko Y, Suraweera AK, Nishimura M, Arakawa T, Takuma T, Mizoguchi I, et al. Differential expression of human beta-defensin 2 in keratinized and non-keratinized oral epithelial lesions: immunohistochemistry and in situ hybridization. Virchows Arch. 2001. 438:248–253.10. Kim YJ, Hong CK, Seo SJ. Regulation of human beta-defensin 3 in human keratinocyte cell lines. Ann Dermatol. 2003. 15:1–7.11. Cho YH. Introduction to urinary tract infections. Korean J Urol. 2006. 47:559–567.12. Fowler JE Jr, Latta R, Stamey TA. Studies of introital colonization in women with recurrent urinary infections. VIII. The role of bacterial interference. J Urol. 1977. 118:296–298.13. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002. 113:Suppl 1A. S5–S13.14. Kaushic CF, Zhou AD, Murdin AD, Wira CR. Effects of estradiol and progesterone on susceptibility and immune response to Chlamydia trachomatis infection in the female reproductive tract. Infect Immun. 2000. 68:4207–4216.15. Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, et al. Progestone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996. 2:1084–1089.16. Brunelli R, Frasca D, Perrone G, Pioli C, Fattorossi A, Zichella L, et al. Hormone replacement therapy affects various immune cell subsets and natural cytotoxicity. Gynecol Obstet Invest. 1996. 41:128–131.17. Raz R. Hormone replacement therapy or prophylaxis in postmenopausal women with recurrent urinary tract infection. J Infect Dis. 2001. 183:Suppl 1. S74–S76.18. Iosif CS, Bekassy Z. Prevalence of genito-urinary symptoms in the last menopause. Acta Obstet Gynecol Scand. 1984. 63:257–260.19. Kjaergaard B, Walter S, Knudsen A, Johansen B, Barlebo H. Treatment with low-dose vaginal estradiol in post-menopausal women. A double blind controlled trial. Ugeskr Laeger. 1990. 152:658–659.20. Cardozo L, Benness C, Abbott D. Low dose oestrogen prophylaxis for recurrent urinary tract infections in elderly women. Br J Obstet Gynaecol. 1998. 105:403–407.21. Schaeffer AJ, Jones JM, Dunn JK. Association of vitro Escherichia coli adherence to vaginal an buccal epithelial cells with susceptibility of women to recurrent urinary-tract infections. N Engl J Med. 1981. 304:1062–1066.22. Linzmeier R, Ho CH, Hoang BV, Ganz T. A 450kb contig of defensin genes on human chromosome 8p23. Gene. 1999. 33:205–211.23. Kaiser V, Diamond G. Expression of mammalian defensin genes. J Leukoc Biol. 2000. 68:779–784.24. Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrohial and cyotoxic peptides of mammalian cells. Annu Rev Immunol. 1993. 11:105–128.25. Jones DF, Bevins CL. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of human bowel. FEBS Lett. 1993. 315:187–192.26. Garcia JR, Krause A, Schulz S, Rodriquez-Jimenez FJ, Kluver E, Adermann K, et al. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001. 15:1819–1821.27. Singh PK, Jia HP, Wiles K, Hesselberth J, Liu L, Consay BA, et al. Production of β-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998. 95:14961–14966.28. Becker MN, Diamond G, Verghese MW, Randell SH. CD14-dependent lipopolysaccharide-induced beta-defensin-2 expression in human tracheobronchial epithelium. J Biol Chem. 2000. 275:29731–29736.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of beta-defensin 1 in Various Skin Tumors
- Modulation of Antimicrobial Peptide Human beta-defensin-3 by Toll-like Receptor Ligands in Vaginal Epithelial Cells
- Effect of estrogen on growth hormone receptor expression of human periodontal ligament cell line
- Expression of Human beta-defensin mRNA in Epidermal Proliferative Diseases
- Expression of Antimicrobial Defensin Peptides of the Human Nasal Mucosa