Korean J Urol.
2011 Aug;52(8):560-565. 10.4111/kju.2011.52.8.560.
Spontaneous Recovery of Cavernous Nerve Crush Injury
- Affiliations
-
- 1Department of Urology, College of Medicine, Hallym University, Seoul, Korea. yang1408@hallym.or.kr
- 2Department of Urology, College of Medicine, Hallym University, Chuncheon, Korea.
- KMID: 1988962
- DOI: http://doi.org/10.4111/kju.2011.52.8.560
Abstract
- PURPOSE
To investigate pathophysiological consequences and spontaneous recovery after cavernous nerve crush injury (CNCI) in a rat model.
MATERIALS AND METHODS
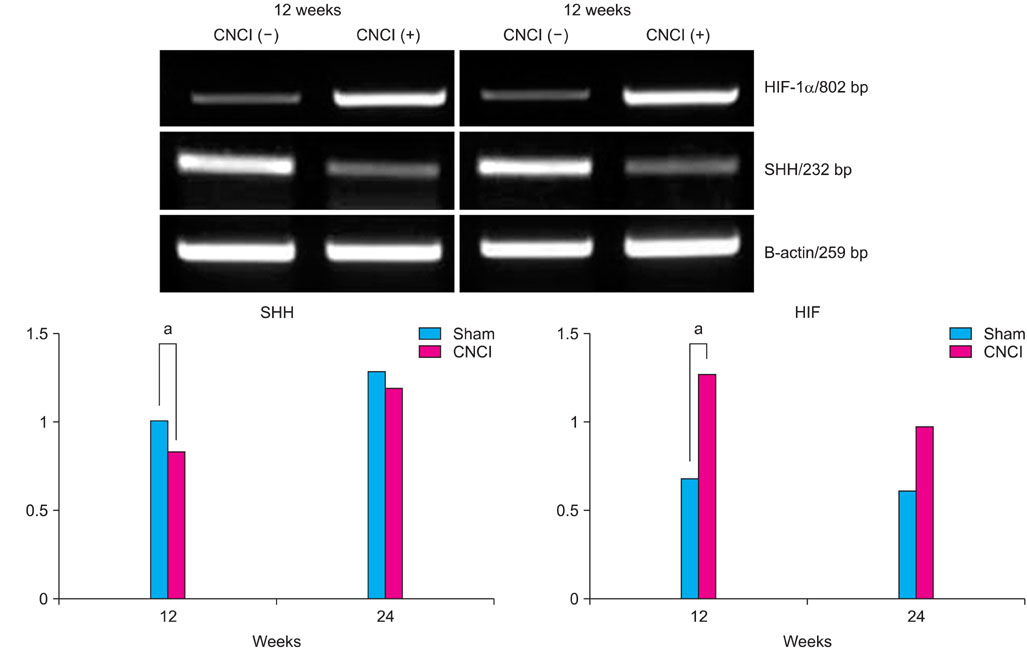
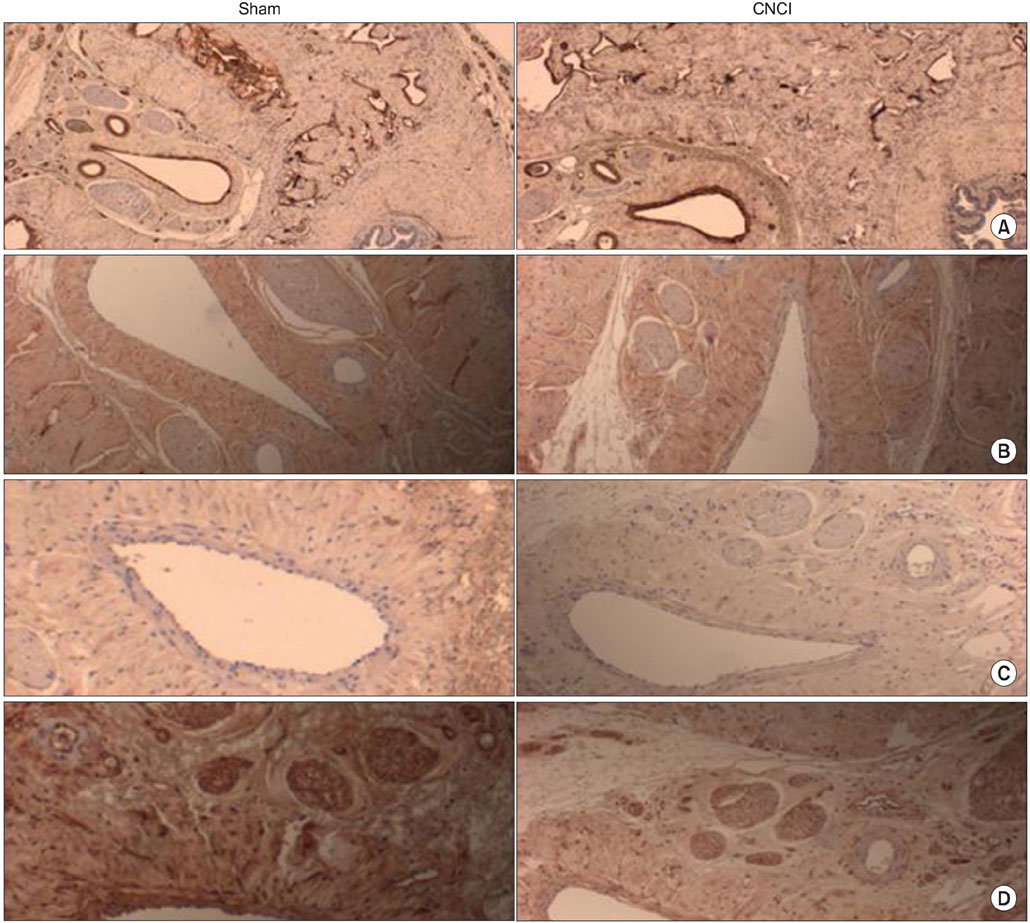
Twenty 4-week-old male Sprague-Dawley rats were divided into the following groups: sham-operated group (n=10) and bilateral CNCI groups (n=10) for two different durations (12 and 24 weeks). At both time points, CN electrical stimulation was used to assess erectile function by measuring the intracavernous pressure. The expression of hypoxia inducible factor (HIF)-1alpha and sonic hedgehog (SHH) was examined in penile tissue. Immunohistochemical staining was performed for nerve growth factor (NGF), endothelial nitric oxide synthase (eNOS), neuronal nitric oxide synthase (nNOS), and smooth muscle alpha-actin.
RESULTS
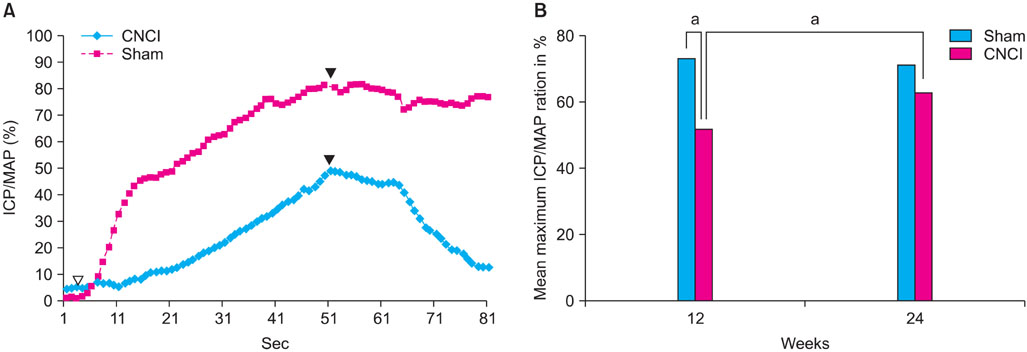
CNCI significantly decreased erectile function at 12 weeks (51.7% vs. 71.9%, mean ICP/BP ratio, p<0.05) and increased the expression of HIF-1alpha and decreased the expression of eNOS, nNOS, and SHH. At 24 weeks, erectile function in the CNCI group was improved with no significant difference versus the sham group (70.5% vs. 63.3%, mean ICP/BP ratio, p<0.05) or the CN group at 12 weeks (51.7% vs. 63.3%, mean ICP/BP ratio, p<0.05). By RT-PCR, the increase in HIF-1alpha and decrease in SHH mRNA was restored at 24 weeks. By immunohistochemistry, the expression of eNOS and nNOS was increased at 24 weeks.
CONCLUSIONS
CN injury induces significantly impaired erectile function and altered gene and protein expression, which suggests that local hypoxic and inflammatory processes may contribute to this change. Significant spontaneous recovery of erectile function was observed at 6 months after CN crush injury.
MeSH Terms
-
Animals
Anoxia
Caves
Electric Stimulation
Erectile Dysfunction
Hedgehog Proteins
Hedgehogs
Humans
Immunohistochemistry
Male
Muscle, Smooth
Nerve Crush
Nerve Growth Factor
Nitric Oxide Synthase Type I
Nitric Oxide Synthase Type III
Rats
Rats, Sprague-Dawley
RNA, Messenger
Salicylamides
Hedgehog Proteins
Nerve Growth Factor
Nitric Oxide Synthase Type I
Nitric Oxide Synthase Type III
RNA, Messenger
Salicylamides
Figure
Cited by 1 articles
-
Is It Possible to Recover Erectile Function Spontaneously after Cavernous Nerve Injury? Time-Dependent Structural and Functional Changes in Corpus Cavernosum Following Cavernous Nerve Injury in Rats
Tae Beom Kim, Min Chul Cho, Jae-Seung Paick, Soo Woong Kim
Korean J Androl. 2012;30(1):31-39. doi: 10.5534/kja.2012.30.1.31.
Reference
-
1. Burnett AL. Rationale for cavernous nerve restorative therapy to preserve erectile function after radical prostatectomy: results from CaPSURE. Urology. 2003. 61:491–497.2. Mulhall JP, Slovick R, Hotaling J, Aviv N, Valenzuela R, Waters WB, et al. Erectile dysfunction after radical prostatectomy: hemodynamic profiles and their correlation with the recovery of erectile function. J Urol. 2002. 167:1371–1375.3. Mulhall J. Neuroimmunophilin ligands protect cavernous nerves after crush injury in the rat: new experimental paradigms. Eur Urol. 2007. 51:1488–1489.4. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006. 50:711–718.5. Angeloni NL, Bond CW, Monsivais D, Tang Y, Podlasek CA. The role of hedgehog-interacting protein in maintaining cavernous nerve integrity and adult penile morphology. J Sex Med. 2009. 6:2480–2493.6. Bond C, Tang Y, Podlasek CA. Neural influences on sonic hedgehog and apoptosis in the rat penis. Biol Reprod. 2008. 78:947–956.7. Podlasek CA, Gonzalez CM, Zelner DJ, Jiang HB, McKenna KE, McVary KT. Analysis of NOS isoform changes in a post radical prostatectomy model of erectile dysfunction. Int J Impot Res. 2001. 13:Suppl 5. S1–S15.8. Jung GW, Kwak JY, Kim JK, Kim DH, Yoon JH. The action mechanism of growth hormone on regeneration of nitric oxide synthase(NOS)-containing nerves after cavernous neurotomy in the rat. Korean J Urol. 1999. 40:1043–1050.9. Klein LT, Miller MI, Buttyan R, Raffo AJ, Burchard M, Devris G, et al. Apoptosis in the rat penis after penile denervation. J Urol. 1997. 158:626–630.10. Chung H, Lee CK, Kim BK, Kim HS, Kim TW, Paick SH, et al. Proteomic analysis of penile protein alterations in a rat model of cavernous nerve injury. Korean J Urol. 2009. 50:498–504.11. Moreland RB, Traish A, McMillin MA, Smith B, Goldstein I, Saenz de Tejada I. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-beta 1 in human corpus cavernosum smooth muscle. J Urol. 1995. 153:826–834.12. Chiappe-Gutierrez M, Kitzmueller E, Labudova O, Fuerst G, Hoeger H, Hardmeier R, et al. mRNA levels of the hypoxia inducible factor (HIF-1) and DNA repair genes in perinatal asphyxia of the rat. Life Sci. 1998. 63:1157–1167.13. Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996. 16:4604–4613.14. Podlasek CA, Meroz CL, Tang Y, McKenna KE, McVary KT. Regulation of cavernous nerve injury-induced apoptosis by sonic hedgehog. Biol Reprod. 2007. 76:19–28.15. Bond C, Tang Y, Podlasek CA. Neural influences on sonic hedgehog and apoptosis in the rat penis. Biol Reprod. 2008. 78:947–956.16. Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996. 274:255–259.17. Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroish T, et al. Unique functions of sonic hedgehog signaling during external genitalia development. Development. 2001. 128:4241–4250.18. Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic Hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002. 247:26–46.19. Podlasek CA, Zelner DJ, Jiang HB, Tang Y, Houston J, McKenna KE, et al. Sonic hedgehog cascade is required for penile postnatal morphogenesis, differentiation, and adult homeostasis. Biol Reprod. 2003. 68:423–438.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Crush Syndrome after Physical Assaults
- Sensory recovery after infraorbital nerve avulsion injury
- Adenoviral vector mediated in vivo gene transfer of BDNF promote functional recovery after facial nerve crush injury
- Expression of Toll-Like Receptor Messenger Ribonucleic Acid in the Distal Facial Nerve After Facial Nerve Injury
- EFFECTS OF SQUARE-PULSE STIMULATION ON FUNCTIONAL RECOVERY FOLLOWING A CRUSH INJURY TO THE SCIATIC NERVE IN RATS