Korean J Sports Med.
2011 Dec;29(2):112-117. 10.5763/kjsm.2011.29.2.112.
Effect of Resistance Exercise Training on Mustn1 mRNA Expression in Rat Skeletal Muscle
- Affiliations
-
- 1Research Institute for Sports Science, Hanyang University, Seoul, Korea. hyriss@hanyang.ac.kr
- KMID: 1987191
- DOI: http://doi.org/10.5763/kjsm.2011.29.2.112
Abstract
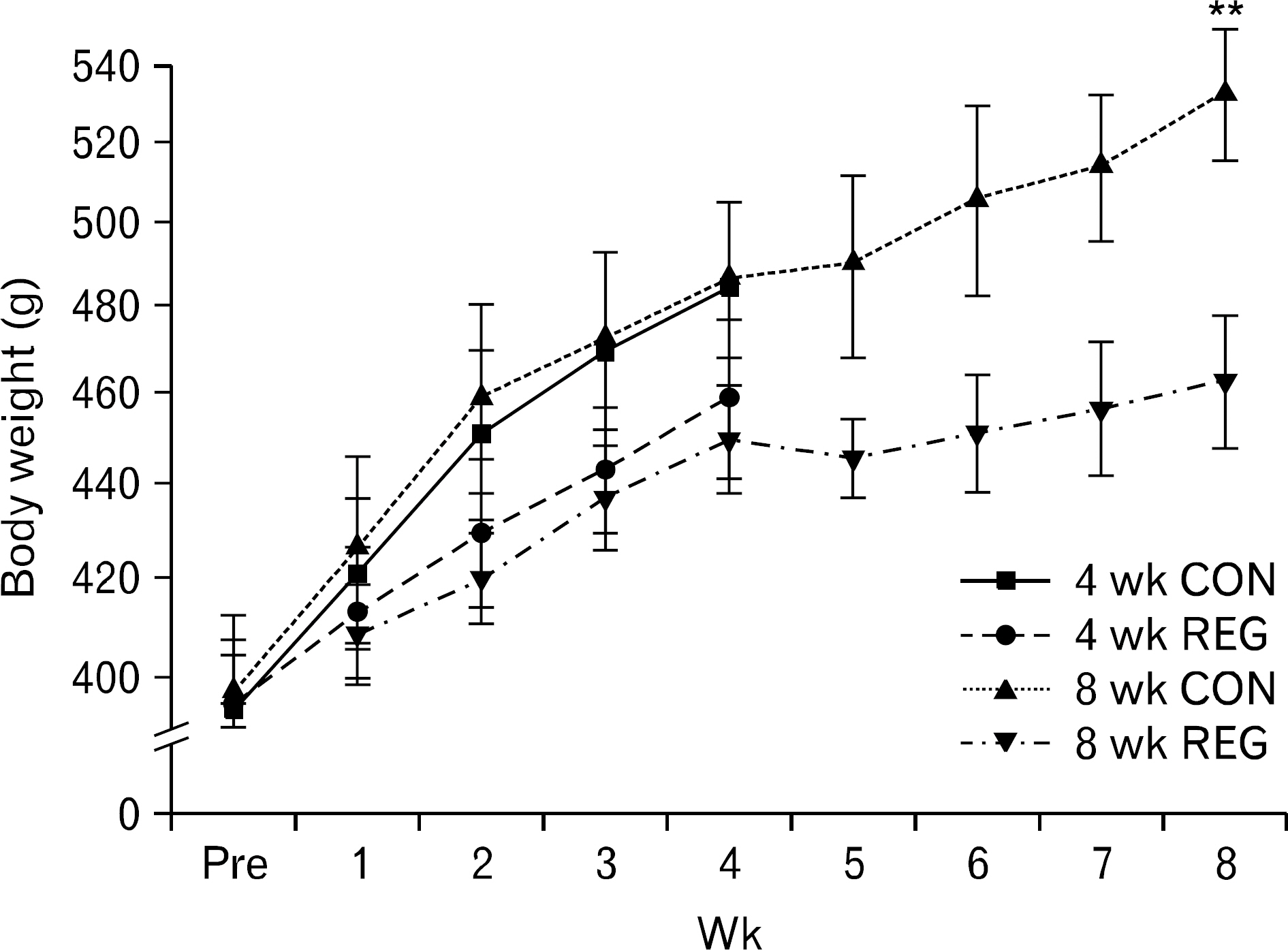
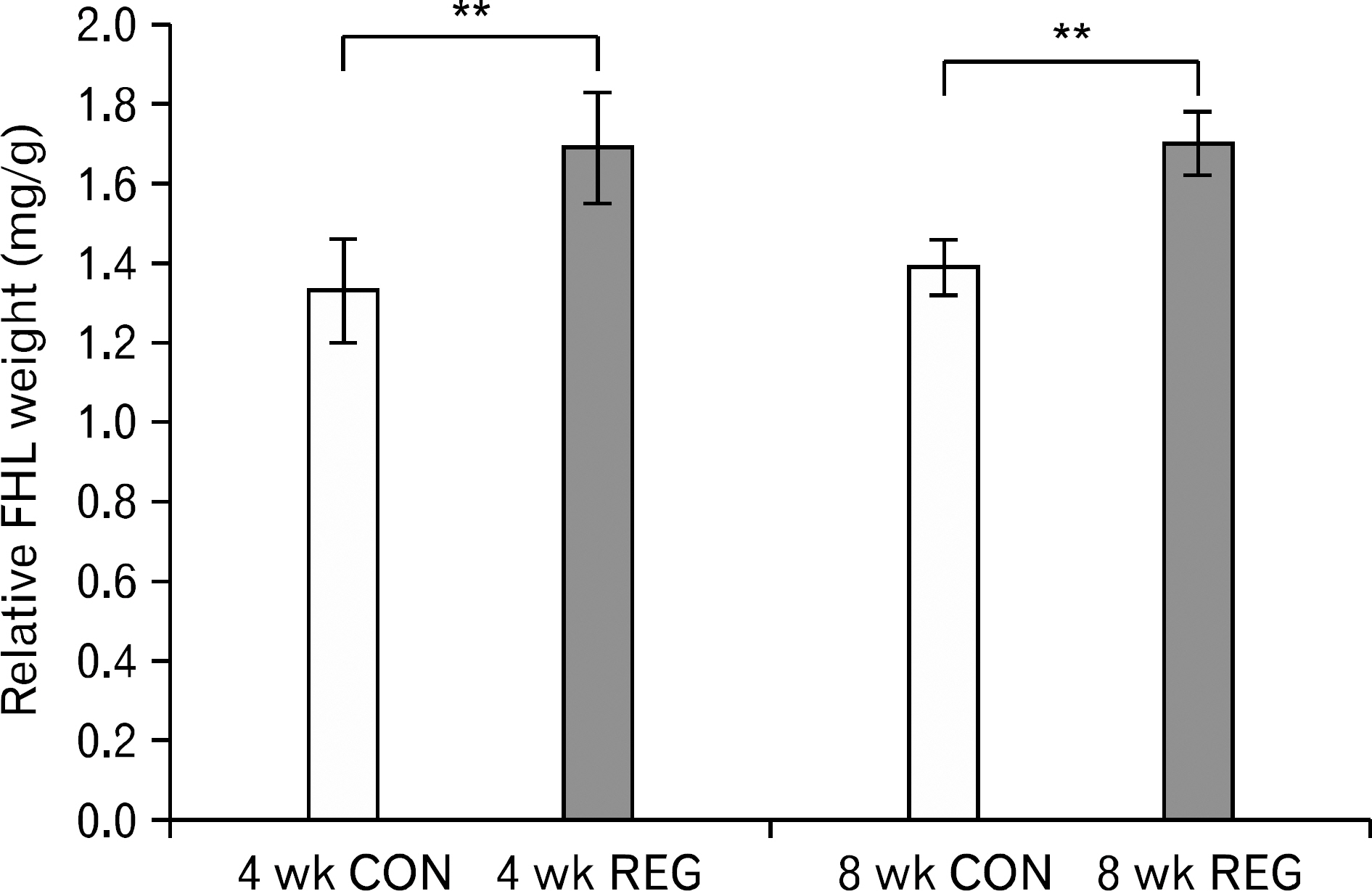
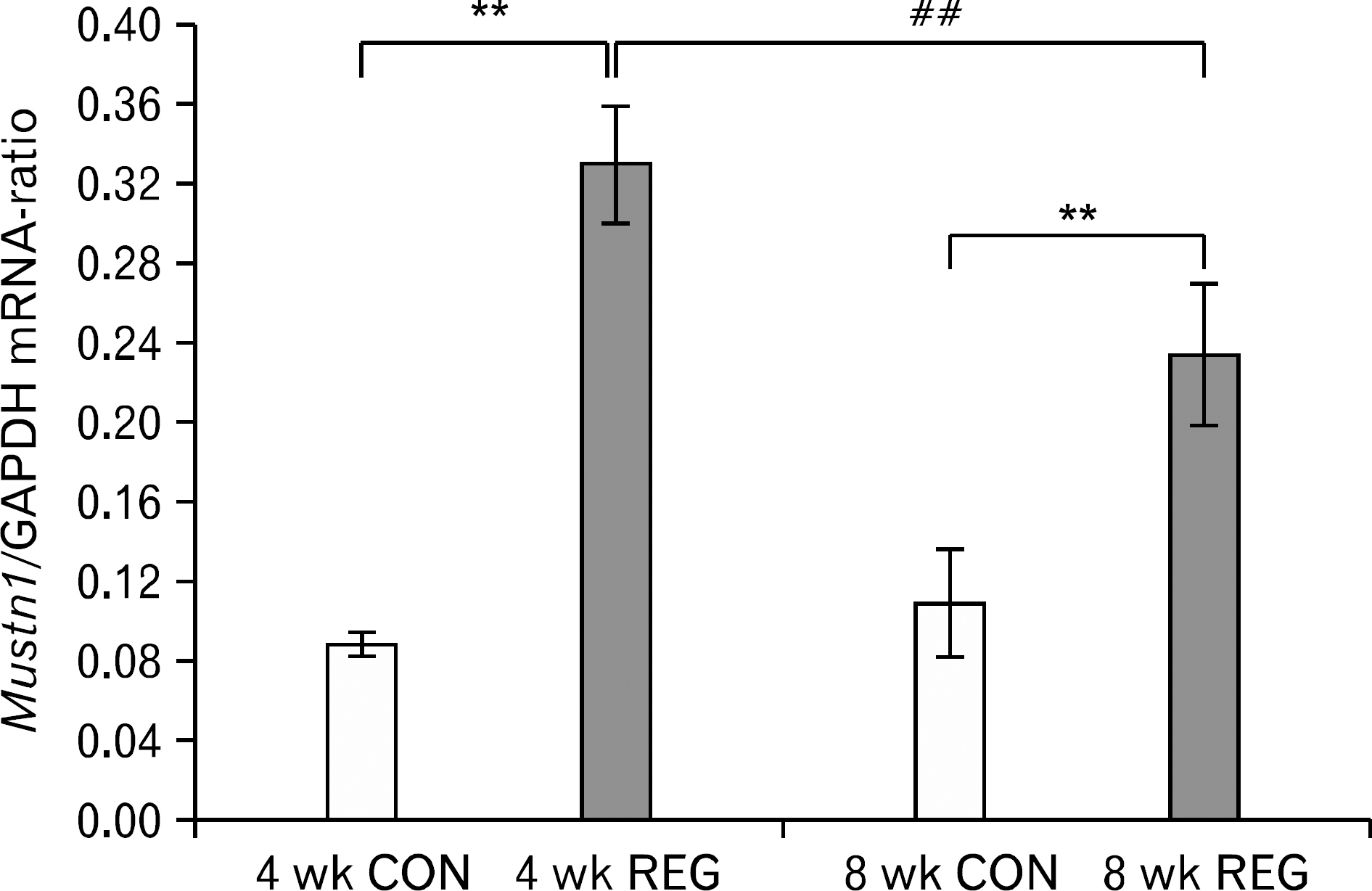
- The aim of this investigation was to determine if resistance exercise improved musculoskeletal embryonic nuclear protein 1 (Mustn1) mRNA expression in skeletal muscle of rat. Thirty-two male Sprague-Dawley rats were separated into sedentary (control group, CON; n=16) and exercise-trained groups (resistance exercise group, REG; n=16). CON and REG subsequently were separated into 4 weeks group (4 weeks CON, 4 weeks REG) and 8 weeks group (8 weeks CON, 8 weeks REG). The rats in the resistance exercise group were trained to climb a 1-m vertical (85 degree incline) ladder with weights secured to their tail, and they climbed the ladder 10 times 3 days per week for 8 weeks progressively. After weeks of exercise, skeletal muscle was taken from the flexor halucis longus. After separating the total ribonucleic acid (RNA) of each group, quantitative polymerase chain reaction was used to analyze RNA quantitatively. After 4 weeks of resistance exercise, Mustn1 mRNA expression increased significantly in REG compared to CON (p<0.001). Additionally, there was a significant increase of Mustn1 mRNA expression in 8 weeks REG compared to 8 weeks CON (p<0.01). Interestingly, there was a significant difference in Mustn1 mRNA between 4 weeks REG and 8 weeks REG (p<0.01). In the REG, Mustn1 mRNA increased by 3.7-fold and 2.1-fold relative to CON, respectively. In conclusion, the resistance training increased Mustn1 mRNA expression in skeletal muscle of rat. This shows that the Mustn1 mRNA expression gives positive effect on myogenesis and muscle regeneration in skeletal muscle of rat results from resistance ladder exercise.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Baechle TR, Earle RW. Essentials of strength training and conditioning. 2nd ed.Champaign (IL): Human Kinetics;2000.2. Fry AC, Allemeier CA, Staron RS. Correlation between percentage fiber type area and myosin heavy chain content in human skeletal muscle. Eur J Appl Physiol Occup Physiol. 1994; 68:246–51.
Article3. Kraemer WJ. Endocrine responses to resistance exercise. Med Sci Sports Exerc. 1988; 20:S152–7.
Article4. Liu C, Hadjiargyrou M. Identification and characterization of the Mustang promoter: regulation by AP-1 during myogenic differentiation. Bone. 2006; 39:815–24.
Article5. Kostek MC, Chen YW, Cuthbertson DJ, et al. Gene ex-pression responses over 24 h to lengthening and shortening contractions in human muscle: major changes in CSRP3, MUSTN1, SIX1, and FBXO32. Physiol Genomics. 2007; 31:42–52.
Article6. Liu C. Functional characterization of Mustn1 during skeletal myogenesis [dissertation]. New York (NY): Stony Brook Univ.;2007.7. Lombardo F, Komatsu D, Hadjiargyrou M. Molecular cloning and characterization of Mustang, a novel nuclear protein expressed during skeletal development and regeneration. FASEB J. 2004; 18:52–61.
Article8. Hadjiargyrou M, Lombardo F, Zhao S, et al. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem. 2002; 277:30177–82.9. Kobayashi J, Mackinnon SE, Watanabe O, et al. The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve. 1997; 20:858–66.
Article10. Hornberger TA Jr, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004; 29:16–31.11. Jung DM. Effects of resistance and hGH injection on muscle function and related gene expression of aged SD rats [dissertation]. Seoul: Graduate School, Kyung Hee Univ.;2006.12. Kwon YE, Chung DM, Park H. Effects of the chronic application of hGH and climbing to the aged female SD rats on their morphological and enzymological properties especially in FHL. Korean J Exerc Nutr. 2004; 8:37–42.13. Lee S, Barton ER, Sweeney HL, Farrar RP. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J Appl Physiol. 2004; 96:1097–104.
Article14. Oh SL, Hwang IG, Oh SD. Effects of regular resistance exercise on IGF-1 and UCP-3 mRNA expression in the rats. J Korea Sports Med. 2009; 27:192–9.15. Gersch RP, Hadjiargyrou M. Mustn1 is expressed during chondrogenesis and is necessary for chondrocyte proliferation and differentiation in vitro. Bone. 2009; 45:330–8.
Article16. Liu C, Gersch RP, Hawke TJ, Hadjiargyrou M. Silencing of Mustn1 inhibits myogenic fusion and differentiation. Am J Physiol Cell Physiol. 2010; 298:C1100–8.
Article17. Zheng Q, Zhang Y, Chen Y, Yang N, Wang XJ, Zhu D. Systematic identification of genes involved in divergent skeletal muscle growth rates of broiler and layer chickens. BMC Genomics. 2009; 10:87.
Article18. Vuocolo T, Byrne K, White J, et al. Identification of a gene network contributing to hypertrophy in callipyge skeletal muscle. Physiol Genomics. 2007; 28:253–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exercise/Resistance Training and Muscle Stem Cells
- Effect of Rhodiola Sachalinensis Administration and Endurance Exercise on Insulin Sensitivity and Expression of Proteins Related with Glucose Transport in Skeletal Muscle of Obese Zucker Rat
- Resistance exercise training‑induced skeletal muscle strength provides protective effects on high‑fat‑diet‑induced metabolic stress in mice
- Effect of Exercise Training on Insulin Sensitivity and Intracellular Glucose Metabolism in Skeletal Muscle of High Fat-fed Rats
- Effect of Exercise on Glucose Metabolism