Korean Circ J.
2012 Oct;42(10):668-673. 10.4070/kcj.2012.42.10.668.
Hypoadiponectinemia in Patients With Paroxysmal Atrial Fibrillation
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Kosin University School of Medicine, Busan, Korea. chatjn@gmail.com
- KMID: 1826463
- DOI: http://doi.org/10.4070/kcj.2012.42.10.668
Abstract
- BACKGROUND AND OBJECTIVES
Adiponectin is an adipose tissue-derived hormone that has beneficial effects on cardiac function and has been reported to be associated with lipid metabolism, glucose metabolism, and insulin resistance. Serum levels of adiponectin are reduced in obese individuals compared with non-obese individuals. Obesity is associated with an increased incidence of atrial fibrillation (AF); however, the role of adiponectin in AF is unclear. The aim of this study is to evaluate the relationship between the plasma adiponectin level and AF.
SUBJECTS AND METHODS
Sixty-one consecutive patients were prospectively enrolled for this study. Subjects were divided into two groups: patients with AF (n=30) and controls (n=31). Laboratory evaluation, including levels of plasma adiponectin, was performed and echocardiographic parameters were measured.
RESULTS
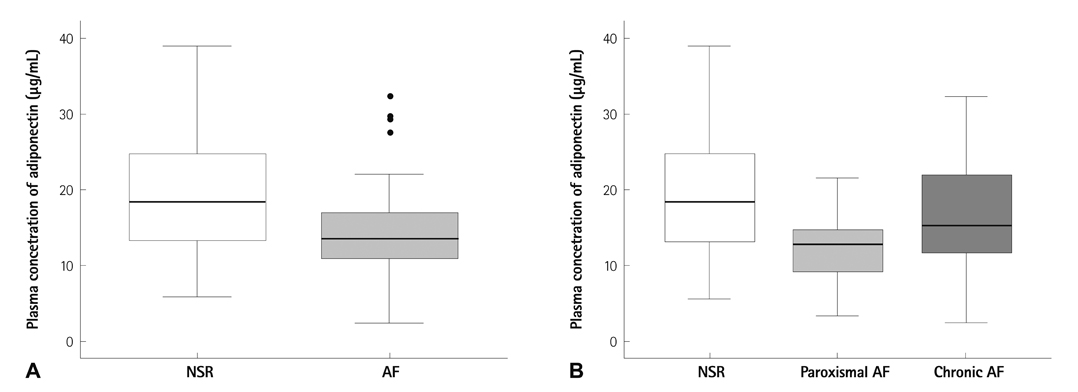
The baseline characteristics were not different between the two groups. The plasma adiponectin level of patients in the AF group was significantly lower than in the control group (14.9+/-7.2 vs. 19.+/-8.9 microg/mL, p<0.05). In addition, when we divided the AF patients into paroxysmal and chronic AF, the plasma adiponectin level was significantly lower in patients with paroxysmal AF, compared with the control group. In multiple binary logistic regression analysis to evaluate the independent predictors for AF, adiponectin and left atrial diameter were strong independent predictors of AF.
CONCLUSION
In this study a lower plasma adiponectin concentration was significantly associated with that of paroxysmal AF. Hypoadiponectinemia can potentially be an important risk factor for AF.
Keyword
MeSH Terms
Figure
Reference
-
1. Goette A, Arndt M, Röcken C, et al. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation. 2000. 101:2678–2681.2. Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001. 104:2886–2891.3. Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005. 26:2083–2092.4. Henningsen KM, Therkelsen SK, Johansen JS, Bruunsgaard H, Svendsen JH. Plasma YKL-40, a new biomarker for atrial fibrillation? Europace. 2009. 11:1032–1036.5. Nicolaou VN, Papadakis JE, Karatzis EN, Dermitzaki SI, Tsakiris AK, Skoufas PD. Impact of the metabolic syndrome on atrial size in patients with new-onset atrial fibrillation. Angiology. 2007. 58:21–25.6. Echahidi N, Mohty D, Pibarot P, et al. Obesity and metabolic syndrome are independent risk factors for atrial fibrillation after coronary artery bypass graft surgery. Circulation. 2007. 116:11 Suppl. I213–I219.7. Han SH, Quon MJ, Kim JA, Koh KK. Adiponectin and cardiovascular disease: response to therapeutic interventions. J Am Coll Cardiol. 2007. 49:531–538.8. Fuster V, Rydén LE, Asinger RW, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Aatrial Fibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. Circulation. 2001. 104:2118–2150.9. McNamara RL, Brass LM, Drozda JP Jr, et al. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Commitee to Develop Data Standards on Atrial Fibrillation). J Am Coll Cardiol. 2004. 44:475–495.10. Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance: endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Soc Echocardiogr. 2007. 20:787–805.11. Celik T, Iyisoy A. A new villain of the village: hypoadiponectinemia. Int J Cardiol. 2009. 137:54–55.12. Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005. 112:1756–1762.13. George J, Patal S, Wexler D, et al. Circulating adiponectin concentrations in patients with congestive heart failure. Heart. 2006. 92:1420–1424.14. McEntegart MB, Awede B, Petrie MC, et al. Increase in serum adiponectin concentration in patients with heart failure and cachexia: relationship with leptin, other cytokines, and B-type natriuretic peptide. Eur Heart J. 2007. 28:829–835.15. Tsutamoto T, Tanaka T, Sakai H, et al. Total and high molecular weight adiponectin, haemodynamics, and mortality in patients with chronic heart failure. Eur Heart J. 2007. 28:1723–1730.16. Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002. 13:134–141.17. Guebre-Egziabher F, Bernhard J, Funahashi T, Hadj-Aissa A, Fouque D. Adiponectin in chronic kidney disease is related more to metabolic disturbances than to decline in renal function. Nephrol Dial Transplant. 2005. 20:129–134.18. Shimano M, Shibata R, Tsuji Y, et al. Circulating adiponectin levels in patients with atrial fibrillation. Circ J. 2008. 72:1120–1124.19. Rienstra M, Sun JX, Lubitz SA, et al. Plasma resistin, adiponectin, and risk of incident atrial fibrillation: the Framingham Offspring Study. Am Heart J. 2012. 163:119–124.20. Kumagai K. Upstream approach for atrial fibrillation. Nihon Yakurigaku Zasshi. 2010. 135:59–61.21. Furuhashi M, Ura N, Higashiura K, et al. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003. 42:76–81.22. Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension. 2000. 35:1270–1277.23. Guo LL, Pan Y, Jin HM. Adiponectin is positively associated with insulin resistance in subjects with type 2 diabetic nephropathy and effects of angiotensin II type 1 receptor blocker losartan. Nephrol Dial Transplant. 2009. 24:1876–1883.24. Yenicesu M, Yilmaz MI, Caglar K, et al. Blockade of the renin-angiotensin system increases plasma adiponectin levels in type-2 diabetic patients with proteinuria. Nephron Clin Pract. 2005. 99:c115–c121.25. Tsukamoto O, Fujita M, Kato M, et al. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 2009. 53:2070–2077.26. Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002. 347:305–313.27. Della Mea P, Lupia M, Bandolin V, et al. Adiponectin, insulin resistance, and left ventricular structure in dipper and nondipper essential hypertensive patients. Am J Hypertens. 2005. 18:30–35.28. Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000. 20:1595–1599.29. Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003. 23:85–89.30. Rossi A, Enriquez-Sarano M, Burnett JC Jr, Lerman A, Abel MD, Seward JB. Natriuretic peptide levels in atrial fibrillation: a prospective hormonal and Doppler-echocardiographic study. J Am Coll Cardiol. 2000. 35:1256–1262.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Paroxysmal Atrial Fibrillation and Sinus Bradycardia due to Coronary Artery Spasm
- A clinical observation on antiarrhythmic efficacy of propafenone for atrial fibrillation
- Clinical significance of serum TSH in euthyroid patients with paroxysmal atrial fibrillation
- The Joint Multicenter Study on the Atrial Fibrillation in Korea
- Soluble ST2 in Paroxysmal Atrial Fibrillation: a New Biomarker that Predicts Recurrence?