J Korean Med Sci.
2004 Jun;19(3):413-418. 10.3346/jkms.2004.19.3.413.
Effects of alpha-Phenyl-N-tert-Butyl Nitrone (PBN)on Brain Cell Membrane Function and Energy Metabolism during Transient Global Cerebral Hypoxia-Ischemia and Reoxygenation-Reperfusion in Newborn Piglets
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. mhlee@smc.samsung.co.kr
- 2Department of Pediatrics, Samsung Cheil Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1786821
- DOI: http://doi.org/10.3346/jkms.2004.19.3.413
Abstract
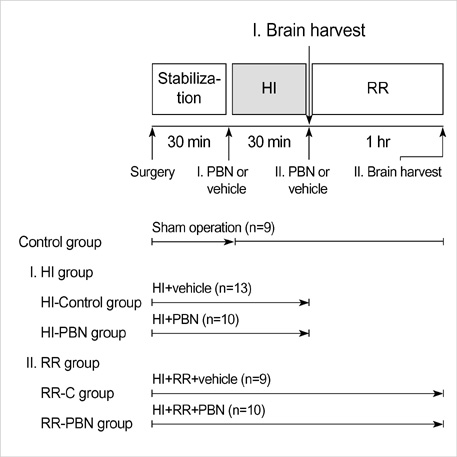
- We sought to know whether a free radical spin trap agent, alpha-phenyl-N-tert-butyl nitrone (PBN) influences brain cell membrane function and energy metabolism during and after transient global hypoxia-ischemia (HI) in the newborn piglets. Cerebral HI was induced by temporary complete occlusion of bilateral common carotid arteries and simultaneous breathing with 8% oxygen for 30 min, followed by release of carotid occlusion and normoxic ventilation for 1 hr (reoxygenationreperfusion, RR). PBN (100 mg/kg) or vehicle was administered intravenously just before the induction of HI or RR. Brain cortex was harvested for the biochemical analyses at the end of HI or RR. The level of conjugated dienes significantly increased and the activity of Na+, K+-ATPase significantly decreased during HI, and they did not recover during RR. The levels of ATP and phosphocreatine (PCr) significantly decreased during HI, and recovered during RR. PBN significantly decreased the level of conjugated dienes both during HI and RR, but did not influence the activity of Na+, K+-ATPase and the levels of ATP and PCr. We demonstrated that PBN effectively reduced brain cell membrane lipid peroxidation, but did not reverse ongoing brain cell membrane dysfunction nor did restore brain cellular energy depletion, in our piglet model of global hypoxic-ischemic brain injury.
Keyword
MeSH Terms
-
Adenosine Triphosphate/metabolism
Animals
Animals, Newborn
*Anoxia
Brain/*drug effects/metabolism/pathology
Cell Membrane/*metabolism
Cerebral Cortex
*Ischemia
Lipid Peroxidation
Na(+)-K(+)-Exchanging ATPase/metabolism
Neuroprotective Agents/*pharmacology
Nitrogen Oxides/*pharmacology
Phosphocreatine/*analogs & derivatives/metabolism
Reperfusion Injury/*drug therapy
Support, Non-U.S. Gov't
Swine
Time Factors
Figure
Reference
-
1. Vannucci RC. Hypoxic-ischemic encephalopathy. Am J Perinatol. 2000. 17:113–120.
Article2. Palmer C. Stevenson DK, Sunshine P, editors. Ischemia-reperfusion injury. Fetal and Neonatal Brain Injury. 1997. Oxford: Oxford University Press;38–58.3. Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res. 1997. 41:599–606.
Article4. Zini I, Tomasi A, Grimaldi R, Vannini V, Agnati LF. Detection of free radicals during brain ischemia and reperfusion by spin trapping and microdialysis. Neurosci Lett. 1992. 138:279–282.
Article5. Siesjö BK, Siesjö P. Mechanisms of secondary brain injury. Eur J Anaesthesiol. 1996. 13:247–268.
Article6. Siesjö BK, Agardh CD, Bengtsson F. Free radicals and brain damage. Cerebrovasc Brain Metab Rev. 1989. 1:165–211.7. Palmer C, Vannucci RC, Towfighi J. Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatr Res. 1990. 27:332–336.
Article8. Palmer C, Towfighi J, Roberts RL, Heitjan DF. Allopurinol administered after inducing hypoxia-ischemia reduces brain injury in 7-day-old rats. Pediatr Res. 1993. 33:405–411.
Article9. Patt A, Harken AH, Burton LK, Rodell TC, Piermattei D, Schorr WJ, Parker NB, Berger EM, Horesh IR, Terada LS. Xanthine oxidase-derived hydrogen peroxide contributes to ischemia reperfusion-induced edema in gerbil brains. J Clin Invest. 1988. 81:1556–1562.
Article10. Liu TH, Beckman JS, Freeman BA, Hogan EL, Hsu CY. Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Physiol. 1989. 256:H589–H593.
Article11. Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996. 27:1124–1129.
Article12. Turrens JF, Crapo JD, Freeman BA. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J Clin Invest. 1984. 73:87–95.
Article13. Chan PH, Longar S, Fishman RA. Protective effects of liposome-entrapped superoxide dismutase on posttraumatic brain edema. Ann Neurol. 1987. 21:540–547.
Article14. Janzen EG, Blackburn BJ. Detection and identification of short-lived free radicals by electron spin resonance trapping technique. J Am Chem Soc. 1968. 90:5909–5910.15. Kalyanaraman B. Detection of toxic free radicals in biology and medicine. Rev Biochem Toxicol. 1982. 4:73–139.16. Cao X, Phillis JW. α-Phenyl-tert-butyl-nitrone reduces cortical infarct and edema in rats subjected to focal ischemia. Brain Res. 1994. 644:267–272.
Article17. Folbergrova J, Zhao Q, Katsura K, Siesjö BK. N-tert-butyl-α-phenylnitrone improves recovery of brain energy state in rats following transient focal ischemia. Proc Natl Acad Sci U.S.A. 1995. 92:5057–5061.18. Kuroda S, Katsura K, Hillered L, Bates TE, Siesjö BK. Delayed treatment with α-phenyl-N-tert-butyl-nitrone (PBN) attenuates secondary mitochondrial dysfunction after transient focal cerebral ischemia in the rat. Neurobiol Dis. 1996. 3:149–157.19. Floyd RA, Carney JM. Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol. 1992. 32:Suppl. S22–S27.
Article20. Janzen EG, Poyer JL, Schaefer CF, Downs PE, DuBose CM. Biological spin trapping. II. Toxicity of nitrone spin traps: dose-ranging in the rat. J Biochem Biophys Methods. 1995. 30:239–247.
Article21. Cheng HY, Liu T, Feuerstein G, Barone FC. Distribution of spin trapping compounds in rat blood and brain: in vivo microdialysis determination. Free Radic Biol Med. 1993. 14:243–250.22. Nakai A, Asakura H, Taniuchi Y, Koshino T, Araki T, Siesjö BK. Effect of α-phenyl-N-tert-butyl nitrone (PBN) on fetal cerebral energy metabolism during intrauterine ischemia and reperfusion in rats. Pediatr Res. 2000. 47:451–456.
Article23. Chang YS, Park WS, Lee M, Kim KS, Shin SM, Choi JH. Effect of hyperglycemia on brain cell membrane function and energy metabolism during hypoxia-ischemia in newborn piglets. Brain Res. 1998. 798:271–280.
Article24. Dugan LL, Choi DW. Excitotoxicity, free radicals, and cell membrane changes. Ann Neurol. 1994. 35:Suppl. S17–S21.
Article25. Palmer C. Hypoxic-ischemic encephalopathy. Therapeutic approaches against microvascular injury, and role of neutrophils, PAF, and free radicals. Clin Perinatol. 1995. 22:481–517.
Article26. Roth SC, Edwards AD, Cady EB, Delpy DT, Wyatt JS, Azzopardi D, Baudin J, Townsend J, Stewart AL, Reynolds EO. Relation between cerebral oxidative metabolism following birth asphyxia and neurodevelopmental outcome and brain growth at one year. Dev Med Child Neurol. 1992. 34:285–295.
Article27. Mayevsky A. Brain NADH redox state monitored in vivo by fiber optic surface fluorometry. Brain Res. 1984. 319:49–68.
Article28. Mishra OP, Delivoria-Papadopoulos M. Lipid peroxidation in developing fetal guinea pig brain during normoxia and hypoxia. Dev Brain Res. 1989. 45:129–135.
Article29. Lynch RE, Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978. 253:4697–4699.
Article30. Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983. 23:239–257.
Article31. Chang YS, Park WS, Lee M, Kim KS, Shin SM, Choi JH. Near infrared spectroscopic monitoring of secondary cerebral energy failure after transient global hypoxia-ischemia in the newborn piglet. Neurol Res. 1999. 21:216–224.
Article32. Park WS, Chang YS, Lee M. Effect of hypothermia on brain cell membrane function and energy metabolism after transient global hypoxiaischemia in the newborn piglet. J Korean Med Sci. 2001. 16:335–341.
Article33. Park WS, Chang YS, Lee M. Effects of hyperglycemia or hypoglycemia on brain cell membrane function and energy metabolism during the immediate reoxygenation-reperfusion period after acute transient global hypoxia-ischemia in the newborn piglet. Brain Res. 2001. 901:102–108.
Article34. Choi CW, Hwang JH, Chang YS, Park WS. Effects of 7-nitroindazole on brain cell membrane function and energy metabolism during transient global cerebral hypoxia-ischemia and reoxygenation-reperfusion in newborn piglets. Korean J Pediatr. 2004. 47:204–209.35. Goplerud JM, Mishra OP, Delivoria-Papadopoulos M. Brain cell membrane dysfunction following acute asphyxia in newborn piglets. Biol Neonate. 1992. 61:33–41.
Article36. Rosenkrantz TS, Kubin J, Mishra OP, Smith D, Delivoria-Papadopoulos M. Brain cell membrane Na+, K+-ATPase activity following severe hypoxic injury in the newborn piglet. Brain Res. 1996. 730:52–57.37. Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985. 65:101–148.
Article38. Hexum TD, Fried R. Effects of superoxide radicals on transport (Na+K) adenosine triphosphatase and protection by superoxide dismutase. Neurochem Res. 1979. 4:73–82.
Article39. Lees GJ. Inhibition of sodium-potassium-ATPase: a potentially ubiquitous mechanism contributing to central nervous system neuropathology. Brain Res Brain Res Rev. 1991. 16:283–300.
Article40. Blumberg RM, Cady EB, Wigglesworth JS, McKenzie JE, Edwards AD. Relation between delayed impairment of cerebral energy metabolism and infarction following transient focal hypoxia-ischaemia in the developing brain. Exp Brain Res. 1997. 113:130–137.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of 7-Nitroindazole on Brain Cell Membrane Function and Energy Metabolism during Transient Global Cerebral Hypoxia-Ischemia and Reoxygenation-Reperfusion in Newborn Piglets
- The Neuroprotective Effect of alpha-phenyl-N-tert-butyl-nitrone (PBN) in the Argon Laser Induced Retinal Ischemia
- Effect of hypothermia on brain cell membrane function and energy metabolism after transient global hypoxia-ischemia in the newborn piglet
- Effects of NG-monomethyl-L-arginine and L-arginine on cerebral hemodynamics and energy metabolism during reoxygenation-reperfusion after cerebral hypoxia-ischemia in newborn piglets
- The effect of hyperglycemia on lipid peroxidation in the global cerebral ischemia of the rat