J Korean Med Sci.
2004 Feb;19(1):69-73. 10.3346/jkms.2004.19.1.69.
Airway Inflammation and Allergen Specific IgE Production May Persist Longer Than Airway Hyperresponsiveness in Mice
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea. shcho@plaza.snu.ac.kr
- 2Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center.
- 3Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- KMID: 1785696
- DOI: http://doi.org/10.3346/jkms.2004.19.1.69
Abstract
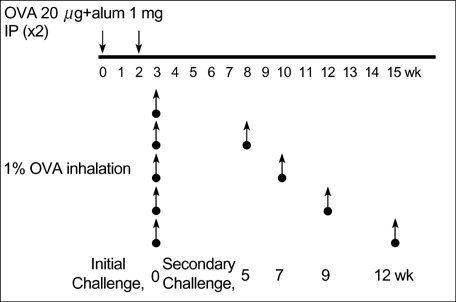
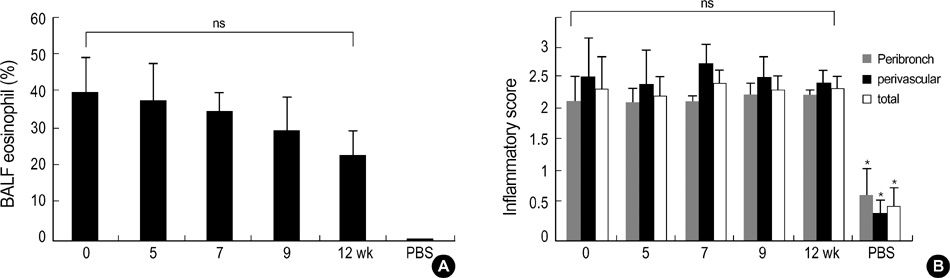
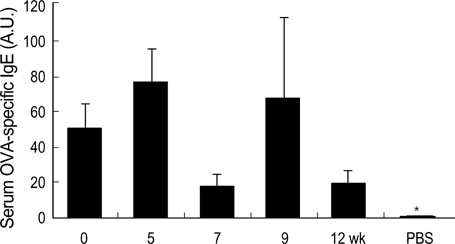
- During the preclinical study of new therapeutic modality, we evaluate whether the treatment can reverse the established asthma phenotypes in animal model. However, few have reported on the long term persistence of asthma phenotypes upon re-challenge with allergen (secondary challenge) in animal model. We evaluated the persistence of asthma phenotypes by secondary challenge at different times in previously challenged murine asthma model. BALB/c mice sensitized by intraperitoneal injections of 20 microgram of ovalbumin and 1 mg of alum on days 1 and 14 were challenged initially by the inhalation of 1% ovalbumin for 30 min on days 21, 22, and 23. Each group of mice was rechallenged at 5, 7, 9, or 12 weeks after the initial challenge. Airway hyperresponsiveness, BAL fluid, airway histology and serum ovalbumin-specific IgE level were evaluated. Airway eosinophilia, airway inflammation and serum ovalbumin-specific IgE production persisted upon secondary allergen challenges at least 12 weeks after the initial challenge. However, airway hyperresponsiveness persisted only until mice were rechallenged 7 weeks after the initial challenge. Airway inflammation and allergen specific IgE production may persist longer than airway hyperresponsiveness in a mouse asthma model of secondary allergen challenge.
Keyword
MeSH Terms
-
Allergens
Animals
Asthma/metabolism/*pathology
Bronchial Hyperreactivity/*diagnosis
Bronchoalveolar Lavage
Bronchoalveolar Lavage Fluid
Female
Immunoglobulin E/*biosynthesis/chemistry
*Inflammation
Lung/pathology
Mice
Mice, Inbred BALB C
Ovalbumin/pharmacology
Phenotype
Respiratory Hypersensitivity/*diagnosis
Respiratory System/pathology
Support, Non-U.S. Gov't
Time Factors
Figure
Cited by 2 articles
-
The Effect of CpG-Oligodeoxynucleotides with Different Backbone Structures and 3' Hexameric Deoxyriboguanosine Run Conjugation on the Treatment of Asthma in Mice
Yoon-Seok Chang, Yoon-Keun Kim, Hyouk-Soo Kwon, Heung-Woo Park, Kyung-Up Min, You-Young Kim, Sang-Heon Cho
J Korean Med Sci. 2009;24(5):860-866. doi: 10.3346/jkms.2009.24.5.860.Influence of the Adjuvants and Genetic Background on the Asthma Model Using Recombinant Der f 2 in Mice
Yoon-Seok Chang, Yoon-Keun Kim, Seong Gyu Jeon, Sae-Hoon Kim, Sun-Sin Kim, Heung-Woo Park, Kyung-Up Min, You-Young Kim, Sang-Heon Cho
Immune Netw. 2013;13(6):295-300. doi: 10.4110/in.2013.13.6.295.
Reference
-
1. Kim YY, Cho SH, Kim WK, Park JK, Song SH, Kim YK, Jee YK, Ha MN, Ahn YO, Lee SI, Min KU. Prevalence of childhood asthma based on questionnaires and methacholine bronchial provocation test in Korea. Clin Exp Allergy. 1997; 27:761–768.
Article2. Beasly R. The burden of asthma with specific reference to the United States. J Allergy Clin Immunol. 2002; 109:Suppl 5. S482–S489.3. Leong KP, Huston DP. Understanding the pathogenesis of allergic asthma using mouse models. Ann Allergy Asthma Immunol. 2001; 87:96–109.
Article4. Institute of laboratory animal resources, commission on life sciences, National research council, USA. Guide for the care and use of laboratory animals. 1996. Washington D.C: National academy press.5. Park YJ, Chang YS, Lee SW, Cho SY, Kim YK, Min KU, Kim YY, Cho SH, Sung YC. The enhanced effect of a hexameric deoxyriboguanosine run conjugation to CpG oligodeoxynucleotides on the protection against allergic asthma. J Allergy Clin Immunol. 2001; 108:570–576.6. Hamelmann E, Schwarze J, Takeda SK, Oshiba A, Larsen GL, Irvin CG, Gelfand E. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997; 156:766–775.
Article7. Tournoy KG, Kips JC, Schou C, Pauwels RA. Airway eosinophilia is not a requirement for allergen-induced airway hyperresponsiveness. Clin Exp Allergy. 2000; 30:79–85.
Article8. Kline JN, Waldschmidt TJ, Businga TR, Lemish JE, Thorne PS, Krieg AM. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J Immunol. 1998; 160:2555–2559.9. Broide D, Schwarze J, Tighe H, Gifford T, Nguyen MD, Malek S, Van Uden J, Martin-Orozco E, Gelfand EW, Raz E. Immunostimulatory DNA sequences inhibit IL-5, eosinophilic inflammation, and airway hyperresponsiveness in mice. J Immunol. 1998; 161:7054–7062.10. Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K. Regulation of murine airway eosinophilia and Th2 cells by antigen-conjugated CpG oligodeoxynucleotides as a novel antigen-specific immunomodulator. J Immunol. 2000; 164:5575–5582.
Article11. Sur S, Wild JS, Choudhury BK, Sur N, Alam R, Klinman DM. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J Immunol. 1999; 162:6284–6293.12. Kanehiro A, Ikemura T, Makela MJ, Lahn M, Joetham A, Dakhama A, Gelfand EW. Inhibition of phosphodiesterase 4 attenuates airway hyperresponsiveness and airway inflammation in a model of secondary allergen challenge. Am J Respir Crit Care Med. 2001; 163:173–184.
Article13. O'Byrne PM. Airway hyperresponsiveness. In : Middletone E., editor. Allergy principles & practice. 1998. 5th ed. St. Louis: Mosby;p. 859–866.14. Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not Interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996; 183:109–117.
Article15. Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airway hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996; 183:195–201.16. Drazen JM, Arm JP, Austen KF. Sorting out the cytokines of asthma. J Exp Med. 1996; 183:1–5.
Article17. Hogan SP, Matthaei KI, Young JM, Koskinen A, Young IG, Foster PS. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J Immunol. 1998; 161:1501–1509.18. Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: Central mediator of allergic asthma. Science. 1998; 282:2258–2261.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of CpG-oligodeoxynucleotides in Chronic Inflammation and Remodeling of Airway in a Murine Model of Bronchial Asthma
- Role of CD8 (+) T Cells in Airway Inflammation and Hyperresponsiveness
- Allergen-induced airway inflammation and its therapeutic intervention
- The Effectiveness of Pimecrolimus in Airway Inflammation and Bronchial Hyperresponsiveness in Murine Asthma Model
- The effects of early allergen/endotoxin exposure on subsequent allergic airway inflammation to allergen in mouse model of asthma