Yonsei Med J.
2007 Apr;48(2):301-307. 10.3349/ymj.2007.48.2.301.
Beneficial Effects of Thiazolidinediones on Diabetic Nephropathy in OLETF Rats
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. cchung@yonsei.ac.kr
- 2Department of Internal Medicine, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 3Department of Anatomy, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 1779535
- DOI: http://doi.org/10.3349/ymj.2007.48.2.301
Abstract
- PURPOSE
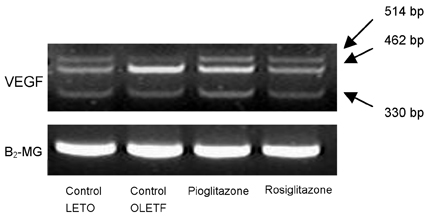
Diabetic nephropathy is the most serious of complications in diabetes mellitus. Thiazolidinedione (TZD) is thought to ameliorate diabetic nephropathy; however, the mechanism underlying this effect has not been elucidated. We hypothesized that the vascular endothelial growth factor (VEGF) participates in the pathogenesis of diabetic nephropathy and that TZD may be beneficial for the treatment of diabetic nephropathy because of the effect it has on VEGF. MATERIALS AND METHODS: 23 Otsuka- Long-Evans-Tokushima-Fatty (OLETF) rats and eight control Long-Evans-Tokushima-Otsuka (LETO) rats were divided into the following four groups: LETO group, control OLETF group, pioglitazone treated group (10mg/kg/day), and rosiglitazone treated group (3mg/kg/day). RESULTS: A progressive increase in urinary protein excretion was observed in the diabetic rats. Glomerular VEGF expression in the control OLETF rats was significantly higher than in the control LETO rats. However, there was a significant reduction in both the glomerular VEGF expression and the VEGF mRNA levels after treatment with pioglitazone and rosiglitazone. The twenty-four hour urine protein levels were significantly decreased in both groups of the treated OLETF rats. CONCLUSION: These results suggest that TZD may have beneficial effects on diabetic nephropathy by reducing the VEGF expression.
Keyword
MeSH Terms
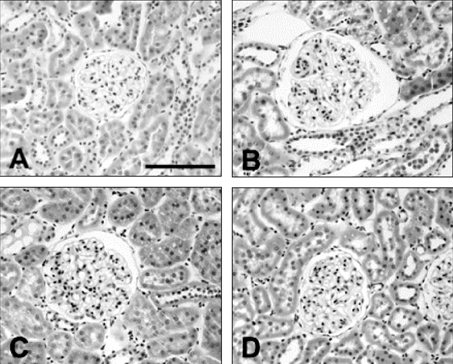
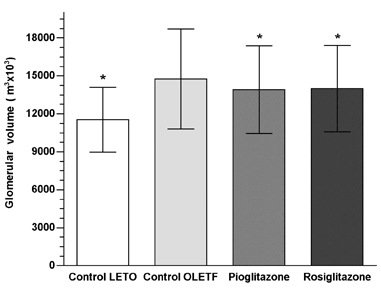
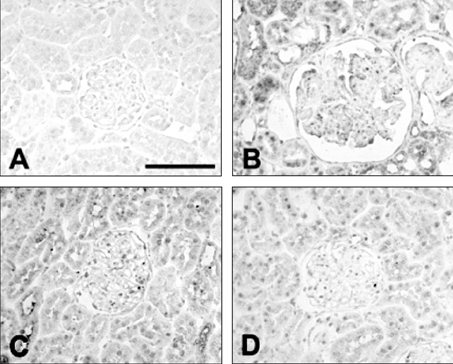
Figure
Reference
-
1. Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, Rizkalla B, et al. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999. 48:2229–2239.2. Tsuchida K, Makita Z, Yamagishi S, Atsumi T, Miyoshi H, Obara S, et al. Suppression of transforming growth factor beta and vascular endothelial growth factor in diabetic nephropathy in rats by a novel advanced glycation end product inhibitor, OPB-9195. Diabetologia. 1999. 42:579–588.3. Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999. 56:794–814.4. Cha DR, Kang YS, Han SY, Jee IH, Han KH, Han JY, et al. Vascular endothelial growth factor is increased during early stage of diabetic nephropathy in type II diabetic rats. J Endocrinol. 2004. 183:183–194.5. de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001. 12:993–1000.6. Imano E, Kanda T, Nakatani Y, Nishida T, Arai K, Motomura M, et al. Effect of troglitazone on microalbuminuria in patients with incipient diabetic nephropathy. Diabetes Care. 1998. 21:2135–2139.7. Nakamura T, Ushiyama C, Osada S, Hara M, Shrimada M, Koide H. Pioglitazone reduces urinary podocyte excretion in type 2 diabetes patients with microalbuminuria. Metabolism. 2001. 50:1193–1196.8. Pistrosch F, Herbrig K, Kindel B, Passauer J, Fischer S, Gross P. Rosiglitazone improves glomerular hyperfiltration, renal endothelial dysfunction, and microalbuminuria of incipient diabetic nephropathy in patients. Diabetes. 2005. 54:2206–2211.9. Onozaki A, Midorikawa S, Sanada H, Hayashi Y, Baba T, Katoh T, et al. Rapid change of glucose concentration promotes mesangial cell proliferation via VEGF: inhibitory effects of thiazolidinedione. Biochem Biophys Res Commun. 2004. 317:24–29.10. Lynch KM, Sellers TS, Gossett KA. Evaluation of an automated pyrogallol red-molybdate method for the measurement of urinary protein in rats. Eur J Clin Chem Clin Biochem. 1996. 34:569–571.11. Lane PH, Steffes MW, Mauer SM. Estimation of glomerular volume: a comparison of four methods. Kidney Int. 1992. 41:1085–1089.12. Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice: nephropathy in patients with type 2 diabetes. N Engl J Med. 2002. 346:1145–1151.13. Ziyadeh FN. The extracellular matrix in diabetic nephropathy. Am J Kidney Dis. 1993. 22:736–744.14. Wang TT, Wu XH, Zhang SL, Chan JS. Effect of glucose on the expression of the angiotensinogen gene in opossum kidney cells. Kidney Int. 1998. 53:312–319.15. Wolf G, Ziyadeh FN. The role of angiotensin II in diabetic nephropathy: Emphasis on nonhemodynamic mechanisms. Am J Kidney Dis. 1997. 29:153–163.16. Lee EY, Shim MS, Kim MJ, Hong SY, Shin YG, Chung CH. Angotensin II receptor blocker attenuates overexpression of vascular endothelial growth factor in diabetic podocytes. Exp Mol Med. 2002. 36:65–70.17. Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002. 51:3090–3094.18. Unemori EN, Ferrara N, Bauer EA, Amento EP. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol. 1992. 153:557–562.19. van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, et al. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997. 95:1030–1037.20. Shulman K, Rosen S, Tognazzi K, Manseau EJ, Brown LF. Expression of vascular permeability factor (VPF/VEGF) is altered in many glomerular diseases. J Am Soc Nephrol. 1996. 7:661–666.21. Horita Y, Miyazaki M, Koji T, Kobayashi N, Shibuya M, Razzaque MS, et al. Expression of vascular endothelial growth factor and its receptors in rats with protein overload nephrosis. Nephrol Dial Transplant. 1998. 13:2519–2528.22. Nyengaard JR, Rasch R. The impact of experimental diabetes mellitus in rats on glomerular capillary number and sizes. Diabetologia. 1993. 36:189–194.23. Henry RR. Thiazolidinediones. Endocrinol Metab Clin North Am. 1997. 26:553–573.24. Yoshimoto T, Naruse M, Nishikawa M, Naruse K, Tanabe A, Seki T, et al. Antihypertensive and vasculo- and renoprotective effects of pioglitazone in genetically obese diabetic rats. Am J Physiol. 1997. 272:E989–E996.25. Nakamura T, Ushiyama C, Suzuki S, Shimada N, Sekizuka K, Ebihara L, et al. Effect of troglitazone on urinary albumin excretion and serum type IV collagen concentrations in Type 2 diabetic patients with microalbuminuria or macroalbuminuria. Diabet Med. 2001. 18:308–313.26. Bakris G, Viberti G, Weston WM, Heise M, Porter LE, Freed MI. Rosiglitazone reduces urinary albumin excretion in type II diabetes. J Hum Hypertens. 2003. 17:7–12.27. Isshiki K, Haneda M, Koya D, Maeda S, Sugimoto T, Kikkawa R. Thiazolidinedione compounds ameliorate glomerular dysfunction independent of their insulin-sensitizing action in diabetic rats. Diabetes. 2000. 49:1022–1032.28. Agarwal R. Anti-inflammatory effects of short-term pioglitazone therapy in men with advanced diabetic nephropathy. Am J Physiol Renal Physiol. 2006. 290:F600–F605.29. Martens FM, Visseren FL, Lemay J, de Koning EJ, Rabelink TJ. Metabolic and additional vascular effects of thiazolidinediones. Drugs. 2002. 62:1463–1480.30. Sakamoto J, Kumura H, Moriyama S, Odaka H, Momose Y, Sugiyama Y, et al. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun. 2000. 278:704–711.31. Hirakata M, Tozawa R, Imura Y, Sugiyama Y. Comparison of the effects of pioglitazone and rosiglitazone on macrophage foam cell formation. Biochem Biophys Res Commun. 2004. 323:782–788.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thiazolidinediones on Insulin Resistance and Insulin Secretion in Obese Diabetic OLETF Rats
- Protective Effects of Lithospermate B on Diabetic Nephropathy in OLETF Rat
- Effects of Rosiglitazone on Inflammation in Otsuka Long-Evans Tokushima Fatty Rats
- Protective Effects of Lithospermic Acid B on Diabetic Nephropathy in OLETF Rats Comparing with Amlodipine and Losartan
- Effect of Eplerenone, a Selective Aldosterone Blocker, on the Development of Diabetic Nephropathy in Type 2 Diabetic Rats