J Korean Diabetes Assoc.
2007 Jan;31(1):33-43. 10.4093/jkda.2007.31.1.33.
Thiazolidinediones on Insulin Resistance and Insulin Secretion in Obese Diabetic OLETF Rats
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Ilsan-Paik Hospital, Inje University College of Medicine, Korea.
- 2Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University of Medicine, Korea.
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Hae-nam Hospital, Korea.
- KMID: 2008094
- DOI: http://doi.org/10.4093/jkda.2007.31.1.33
Abstract
-
BACKGROUND: Thiazolidinediones are synthetic peroxisome proliferator-activated receptor-gamma agonists that decrease insulin resistance but, as in vitro and in vivo studies suggest, may have direct beneficial effects on pancreatic beta cells. Here, we investigated the effects of thiazolidinediones (TZDs) on the insulin resistance, beta-cell mass and insulin secretion in obese diabetic OLETF rats.
METHODS
We studied insulin resistance (by hyperinsulinemic euglycemic clamp) and insulin secretion (by hyperglycemic clamp) in TZDs administered OLETF and LETO rats. Histologic alterations of the islets were observed and beta-cell mass was also measured by point counting method.
RESULTS
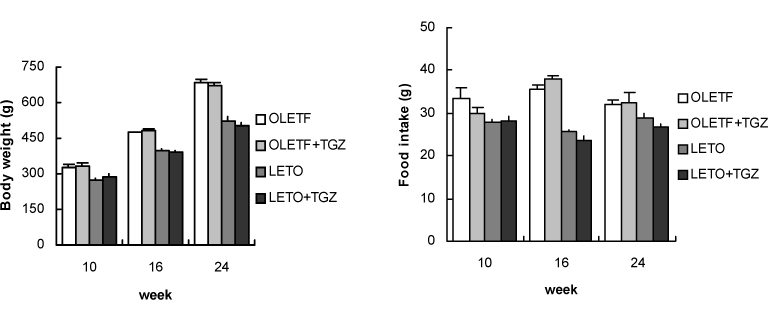
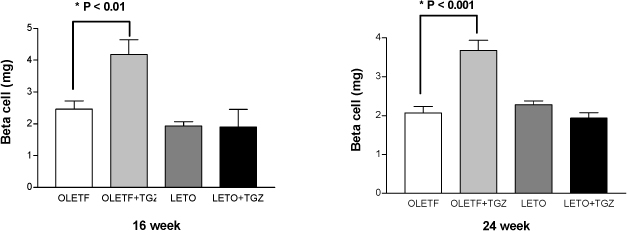
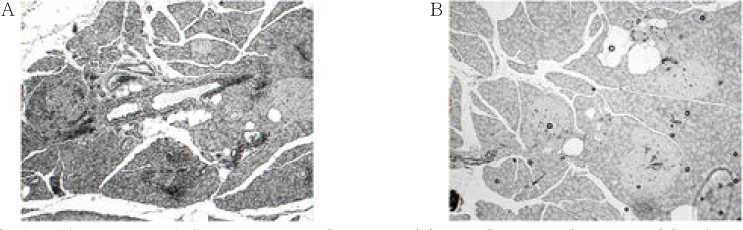
Chronic administration of troglitazone (TGZ, 0.15%) or pioglitazone (PGZ, 0.02%) prevented the development of glucose intolerance in OLETF rats, as assessed by oral glucose tolerance test. There was significant difference in submaximal glucose infusion rate between TGZ-treated and untreated OLETF rats during euglycemic clamp studies at 24 weeks of age. At 16 and 24 weeks of ages, beta-cell mass significantly increased in TGZ-treated OLETF rats compared to untreated animals. At 19 weeks and 30 weeks of age, first-phase insulin secretion was not different in PGZ-treated OLETF rats from untreated OLETF rats during hyperglycemic clamp study. At 30 weeks of age, late-phase insulin secretion was decreased in PGZ-treated OLETF rats compared to untreated OLETF rats. The expression of alpha-smooth muscle actin, a marker of activated pancreatic stellate cells that are involved in the fibrosis of the pancreas, in the islets was suppressed by TGZ treatment at 24 weeks of age.
CONCLUSION
The treatment of TGZ prevented the development of diabetes, and increased insulin sensitivity and pancreatic beta-cell mass in OLETF rats. These results might be related with the suppression of pancreatic stellate cells. Insulin secretion was not affected by PGZ treatment.
MeSH Terms
Figure
Reference
-
1. Mitrakou A, Kelley D, Mokan M, Veneman T, Pangburn T, Reilly J, Gerich J. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med. 1992. 326:22–29.2. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999. 104:787–794.3. Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985. 4:110–125.4. Shimabukuro M, Zhou YT, Lee Y, Unger RH. Troglitazone lowers islet fat and restores beta cell function of Zucker diabetic fatty rats. J Biol Chem. 1998. 273:3547–3550.5. Diani AR, Sawada G, Wyse B, Murray FT, Khan M. Pioglitazone preserves pancreatic islet structure and insulin secretory function in three murine models of type 2 diabetes. Am J Physiol Endocrinol Metab. 2004. 286:E116–E122.6. Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985. 34:222–234.7. Matsuda K, Okamoto Y, Tsuura Y, Kato S, Miura T, Tsuda K, Horikoshi H, Ishida H, Seino Y. Effects of troglitazone (CS-045) on insulin secretion in isolated rat pancreatic islets and HIT cells: an insulinotropic mechanism distinct from glibenclamide. Diabetologa. 1995. 38:24–30.8. Ohtani K, Shimizu H, Tanaka Y, Sato N, Mori M. Pioglitazone hydrochloride stimulates insulin secretion in HIT-T 15 cells by inducing Ca2+ influx. Endocrinol. 1996. 150:107–111.9. de Souza CJ, Yu JH, Robinson DD, Ulrich RG, Meglasson MD. Insulin secretory defect in Zucker fa/fa rats is improved ameliorating insulin resistance. Diabetes. 1995. 44:984–991.10. Miyazaki Y, Matsuda M, Defronzo RA. Dose-response effect of pioglitazone on insulin sensitivity and insulin secretion in type 2 diabetes. Diabetes care. 2002. 25:517–523.11. Juhl CB, Hollingdal M, Porksen N, Prange A, Lonnqvist F, Schmitz O. Influence of rosiglitazone treatment on beta-cell function in type 2 diabetes: evidence of an increased ability of glucose to entrain high-frequency insulin pulsatility. J Clin Endocrinol Metab. 2003. 88:3794–3800.12. Sturis J, Pugh WL, Tang J, Polonsky KS. Prevention of diabetes does not completely prevent insulin secretory defects in the ZDF rat. Am J Physiol. 1995. 269:E786–E792.13. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications, Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992. 41:1422–1428.14. Montana E, Bonner-Weir S, Weir GC. β-cell mass and growth after syngeneic islet cell transplantation in normal and streptozotocin diabetic C57BL/6 mice. J Clin Invest. 1993. 91:780–787.15. Koh EH, Kim MS, Park JY, Kim HS, Youn JY, Park HS, Youn JH, Lee KU. Peroxisome proliferator-activated receptor (PPAR)-alpha activation prevents diabetes in OLETF rats: comparison with PPAR-gamma activation. Diabetes. 2003. 52:2331–2337.16. Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. J Clin Invest. 2000. 106:467–472.17. Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, Pirola RC, McCaughan GW, Ramm GA, Wilson JS. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999. 155:1087–1095.18. Kruse ML, Hildebrand PB, Timke C, Folsche UR, Schmidt WE. TGF beta1 autocrine growth control in isolated pancreatic fibroblastoid cells/stellate cells in vitro. Regul Pept. 2000. 90:47–52.19. Masamune A, Kikuta K, Satoh M, Sakai Y, Satoh A, Shimosegawa T. Ligands of peroxisome proliferator-activated receptor-gamma block activation of pancreatic stellate cells. J Biol Chem. 2002. 277:141–147.20. Jaster R, Lichte P, Fitzner B, Brock P, Glass A, Karopka T, Gierl L, Koczan D, Thiesen HJ, Sparmann G, Emmrich J, Liebe S. Peroxisome proliferator-activated receptor gamma overexpression inhibits pro-fibrogenic activities of immortalised rat pancreatic stellate cells. J Cell Mol Med. 2005. 9:670–682.21. van Westerloo DJ, Florquin S, de Boer AM, Daalhuisen J, de Vos AF, Bruno MJ, van der Poll T. Therapeutic effects of troglitazone in experimental chronic pancreatitis in mice. Am J Pathol. 2005. 166:721–728.22. Im SS, Kim JW, Kim TH, Song XL, Kim SY, Kim HI, Ahn YH. Identification and characterization of peroxisome proliferator response element in the mouse GLUT2 promoter. Exp Mol Med. 2005. 37:101–110.23. Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-α, -β and -γ in the adult rat. Endocrinology. 1996. 137:354–366.24. Dubois M, Pattou F, Kerr-Conte J, Gmyr V, Vandewalle B, Desreumaux P, Auwerx J, Schoonjans K, Lefebvre J. Expression of peroxisome proliferator-activated receptor γ(PPARγ) in normal human pancreatic islet cells. Diabetologia. 2000. 43:1165–1169.25. Nakamichi Y, Kikuta T, Ito E, Ohara-Imaizumi M, Nishiwaki C, Ishida H, Nagamatsu S. PPAR-gamma overexpression suppresses glucose-induced proinsulin biosynthesis and insulin release synergistically with pioglitazone in MIN6 cells. Biochem Biophys Res Commun. 2003. 306:832–836.26. Ovalle F, Bell D. Effect of rosiglitazone vs insulin on pancreatic beta cell function of subjects with type 2 diabetes mellitus. Diabetes. 2003. 52:Suppl 1. A130.27. Zhang B, Saku K, Arakawa K. Quantification of the effects of troglitazone on insulin sensitivity and beta-cell function in Watanabe heritable hyperlipidemic rabbits: a minimal model analysis. Metabolism. 1997. 46:273–281.28. Kim SH, Abbasi F, Chu JW, McLaughlin TL, Lamendola C, Polonsky KS, Reaven GM. Rosiglitazone reduces glucose-stimulated insulin secretion rate and increases insulin clearance in nondiabetic, insulin-resistant individuals. Diabetes. 2005. 54:2447–2452.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Insulin Resistance and Insulin Resistance Syndrome
- Effects of Rosiglitazone on Inflammation in Otsuka Long-Evans Tokushima Fatty Rats
- Taurine-Mediated Restoration of Glucose Sensitivity of Pancreatic Beta Cells in OLETF Rats
- Oxidative Stress Causes Vascular Insulin Resistance in OLETF Rat Through Increased IRS-1 Degradation
- Pathogenetic Heterogeneity of Type 2 Diabetes Mellitus in Korea