Korean Diabetes J.
2010 Jun;34(3):191-199. 10.4093/kdj.2010.34.3.191.
Effects of Rosiglitazone on Inflammation in Otsuka Long-Evans Tokushima Fatty Rats
- Affiliations
-
- 1Department of Internal Medicine, Ulsan University Hospital, Ulsan University Collage of Medicine, Ulsan, Korea. es10@unitel.co.kr
- 2Biomedical Research Center, Ulsan University Hospital, Ulsan, Korea.
- 3Department of Biological Sciences, University of Ulsan, Ulsan, Korea.
- KMID: 2222375
- DOI: http://doi.org/10.4093/kdj.2010.34.3.191
Abstract
- BACKGROUND
Inflammation plays a role in the response to metabolic stress in type 2 diabetes. However, the effects of rosiglitazone on inflammation of skeletal muscle have not been fully examined in type 2 diabetes.
METHODS
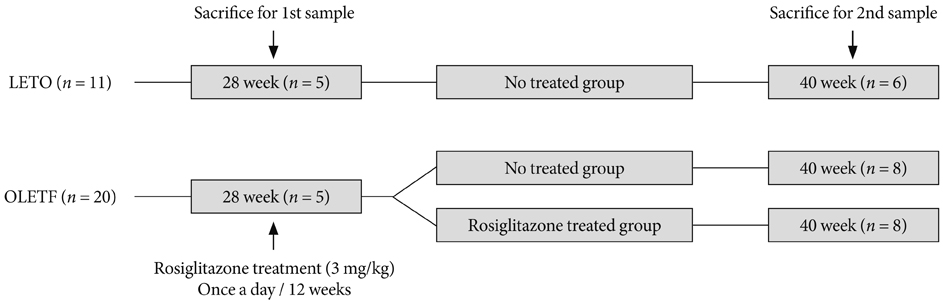
We investigated the effects of the insulin-sensitizing anti-diabetic agent, rosiglitazone, on the progression of skeletal muscle inflammation in Otsuka Long-Evans Tokushima Fatty (OLETF) type 2 diabetic rats. We examined the expression of serologic markers (serum glucose, insulin and free fatty acid) and inflammatory cytokines (tumor-necrosis factor-alpha, interleukin [IL]-1beta and IL-6) in OLETF rats from early to advanced diabetic stage (from 28 to 40 weeks of age).
RESULTS
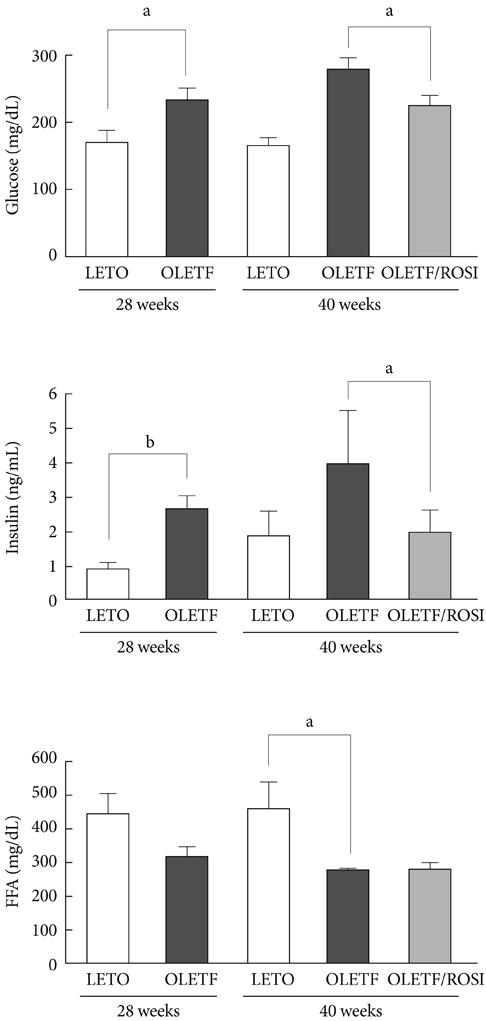
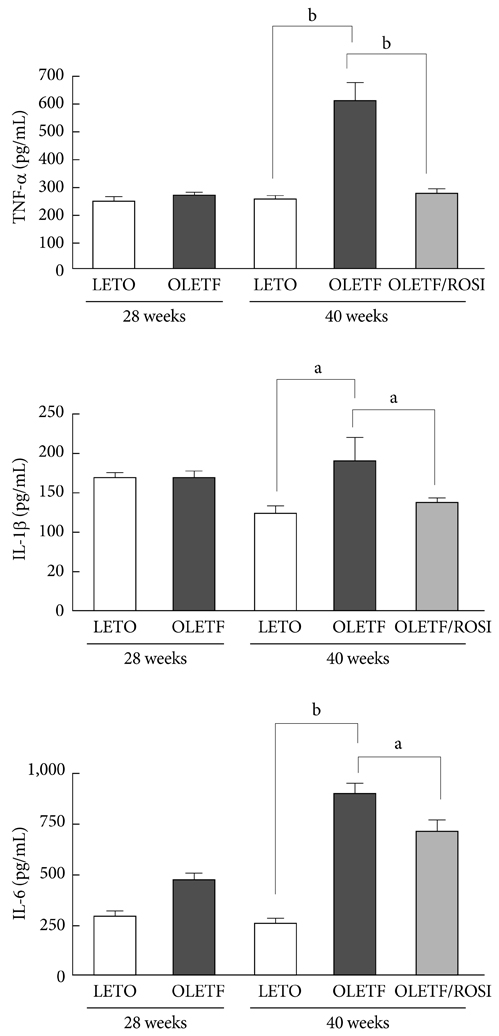
Serum glucose and insulin concentrations were significantly decreased in rosiglitazone-treated OLETF rats compared to untreated OLETF rats. Rosiglitazone treatment significantly decreased the concentrations of serum inflammatory cytokines from 28 to 40 weeks of age. The mRNA expression of various cytokines in skeletal muscle was reduced in rosiglitazone-treated OLETF rats compared with untreated OLETF rats. Furthermore, rosiglitazone treatment resulted in the downregulation of ERK1/2 phosphorylation and NF-kappaB expression in the skeletal muscle of OLETF rats.
CONCLUSION
These results suggest that rosiglitazone may improve insulin sensitivity with its anti-inflammatory effects on skeletal muscle.
Keyword
MeSH Terms
-
Animals
Cytokines
Diabetes Mellitus, Type 2
Down-Regulation
Glucose
Inflammation
Insulin
Insulin Resistance
Interleukins
Muscle, Skeletal
NF-kappa B
Phosphorylation
Rats
Rats, Inbred OLETF
RNA, Messenger
Stress, Physiological
Thiazolidinediones
Cytokines
Glucose
Insulin
Interleukins
NF-kappa B
RNA, Messenger
Thiazolidinediones
Figure
Reference
-
1. Donath MY, Storling J, Berchtold LA, Billestrup N, Mandrup-Poulsen T. Cytokines and beta-cell biology: from concept to clinical translation. Endocr Rev. 2008. 29:334–350.2. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006. 116:1793–1801.3. Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000. 11:327–332.4. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005. 11:183–190.5. Winkler G, Salamon F, Harmos G, Salamon D, Speer G, Szekeres O, Hajos P, Kovacs M, Simon K, Cseh K. Elevated serum tumor necrosis factor-alpha concentrations and bioactivity in type 2 diabetics and patients with android type obesity. Diabetes Res Clin Pract. 1998. 42:169–174.6. Mishima Y, Kuyama A, Tada A, Takahashi K, Ishioka T, Kibata M. Relationship between serum tumor necrosis factor-alpha and insulin resistance in obese men with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2001. 52:119–123.7. Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle: relationship to insulin resistance. J Clin Invest. 1996. 97:1111–1116.8. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996. 271:665–668.9. Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005. 54:2939–2945.10. Bouzakri K, Zierath JR. MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-alpha-induced insulin resistance. J Biol Chem. 2007. 282:7783–7789.11. Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998. 391:82–86.12. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998. 391:79–82.13. Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al-Haddad W, Dhindsa S, Dandona P. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004. 89:2728–2735.14. Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications: Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992. 41:1422–1428.15. Nagaraju K, Raben N, Merritt G, Loeffler L, Kirk K, Plotz P. A variety of cytokines and immunologically relevant surface molecules are expressed by normal human skeletal muscle cells under proinflammatory stimuli. Clin Exp Immunol. 1998. 113:407–414.16. Friedman JE, Kirwan JP, Jing M, Presley L, Catalano PM. Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes. 2008. 57:606–613.17. Sriwijitkamol A, Christ-Roberts C, Berria R, Eagan P, Pratipanawatr T, DeFronzo RA, Mandarino LJ, Musi N. Reduced skeletal muscle inhibitor of kappaB beta content is associated with insulin resistance in subjects with type 2 diabetes: reversal by exercise training. Diabetes. 2006. 55:760–767.18. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995. 95:2409–2415.19. Sethi JK, Hotamisligil GS. The role of TNF alpha in adipocyte metabolism. Semin Cell Dev Biol. 1999. 10:19–29.20. Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, Flanigan A, Murthy S, Lazar MA, Wu GD. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999. 104:383–389.21. Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001. 169:453–459.22. Rasouli N, Raue U, Miles LM, Lu T, Di Gregorio GB, Elbein SC, Kern PA. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab. 2005. 288:E930–E934.23. Staels B. Metformin and pioglitazone: Effectively treating insulin resistance. Curr Med Res Opin. 2006. 22:Suppl 2. S27–S37.24. Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001. 108:437–446.25. Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001. 293:1673–1677.26. Rohl M, Pasparakis M, Baudler S, Baumgartl J, Gautam D, Huth M, De Lorenzi R, Krone W, Rajewsky K, Bruning JC. Conditional disruption of IkappaB kinase 2 fails to prevent obesity-induced insulin resistance. J Clin Invest. 2004. 113:474–481.27. Ghanim H, Dhindsa S, Aljada A, Chaudhuri A, Viswanathan P, Dandona P. Low-dose rosiglitazone exerts an antiinflammatory effect with an increase in adiponectin independently of free fatty acid fall and insulin sensitization in obese type 2 diabetics. J Clin Endocrinol Metab. 2006. 91:3553–3558.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Response: Effects of Rosiglitazone on Inflammation in Otsuka Long-Evans Tokushima Fatty Rats (Korean Diabetes J 2010;34:191-9)
- Letter: Effects of Rosiglitazone on Inflammation in Otsuka Long-Evans Tokushima Fatty Rats (Korean Diabetes J 2010;34:191-9)
- Beneficial Effects of Aerobic Exercise Training Combined with Rosiglitazone on Glucose Metabolism in Otsuka Long Evans Tokushima Fatty Rats
- Femoral bone structure in Otsuka Long-Evans Tokushima Fatty rats
- Beneficial Effects of Thiazolidinediones on Diabetic Nephropathy in OLETF Rats