Korean J Physiol Pharmacol.
2012 Jun;16(3):211-217. 10.4196/kjpp.2012.16.3.211.
Activation of the cGMP/Protein Kinase G Pathway by Nitric Oxide Can Decrease TRPV1 Activity in Cultured Rat Dorsal Root Ganglion Neurons
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 110-799, Korea.
- 2Department of Physiology and Biophysics, Inha University College of Medicine, Incheon 402-751, Korea. kwak1014@inha.ac.kr
- KMID: 1768016
- DOI: http://doi.org/10.4196/kjpp.2012.16.3.211
Abstract
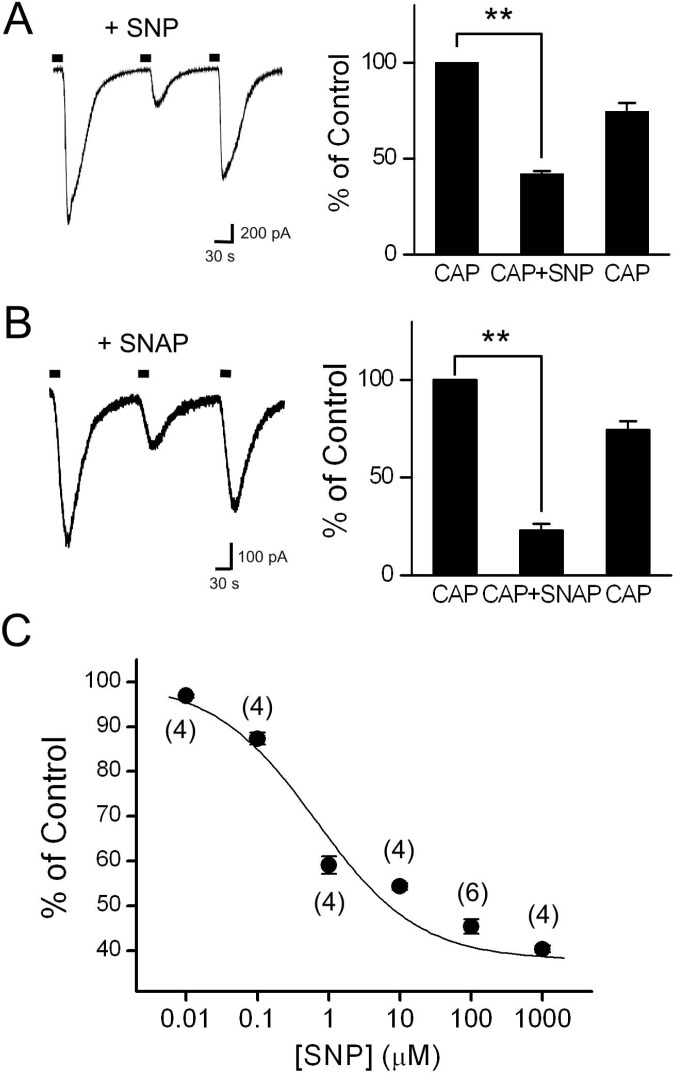
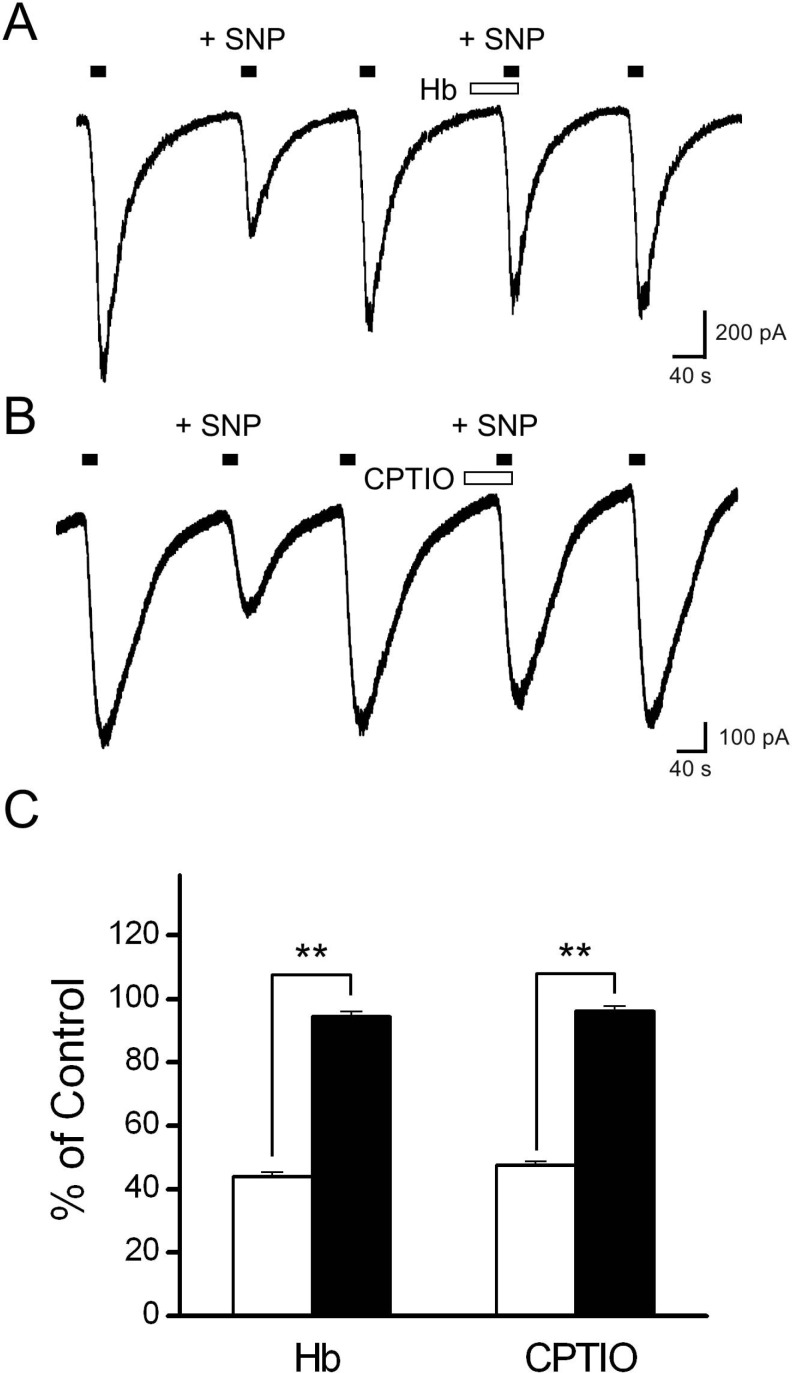
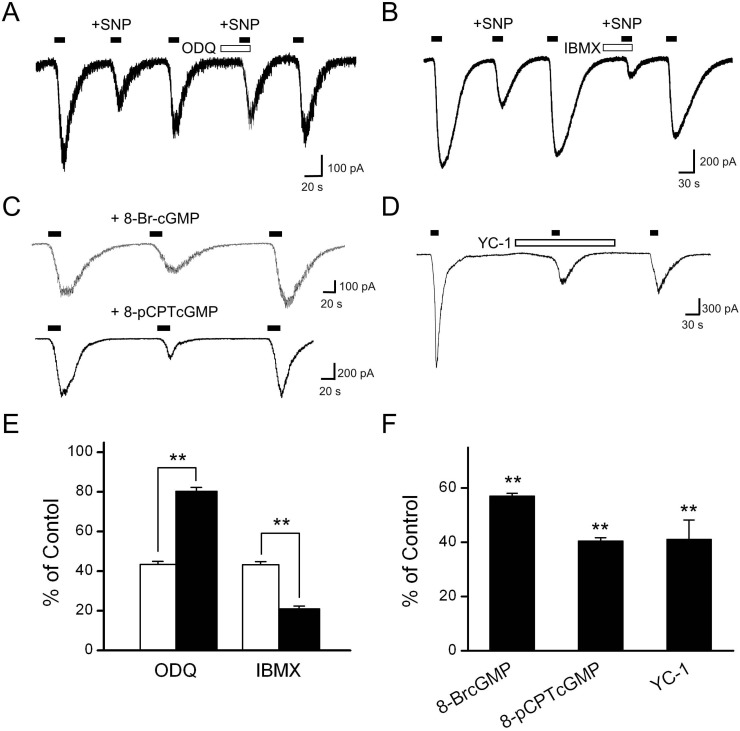
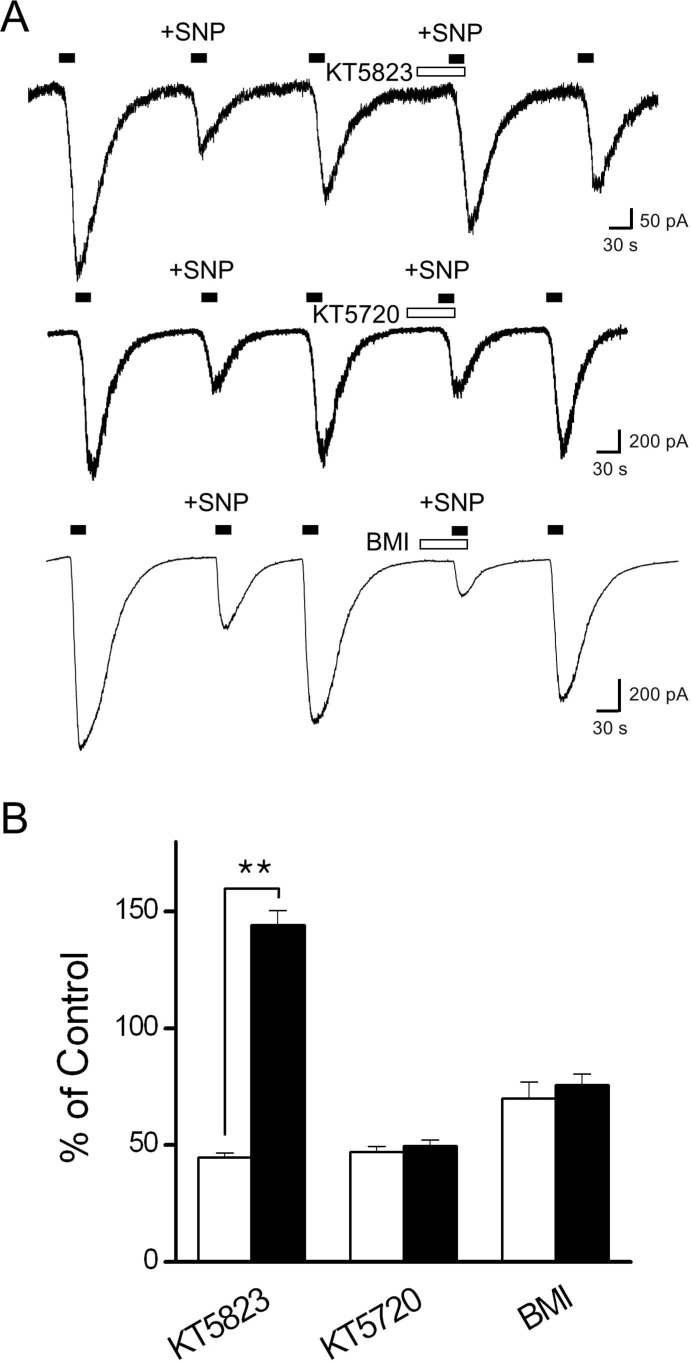
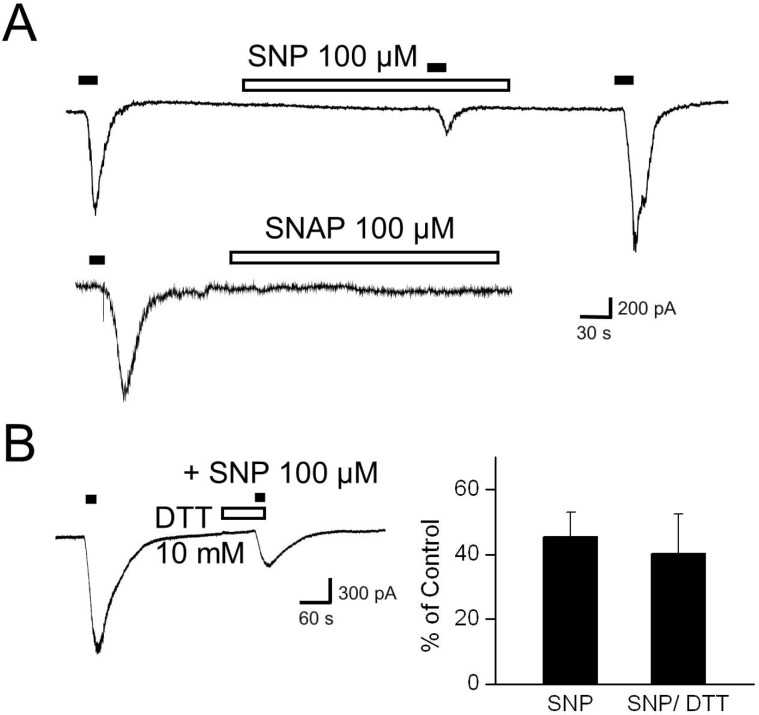
- Recent studies have demonstrated that nitric oxide (NO) activates transient receptor potential vanilloid subtype 1 (TRPV1) via S-nitrosylation of the channel protein. NO also modulates various cellular functions via activation of the soluble guanylyl cyclase (sGC)/protein kinase G (PKG) pathway and the direct modification of proteins. Thus, in the present study, we investigated whether NO could indirectly modulate the activity of TRPV1 via a cGMP/PKG-dependent pathway in cultured rat dorsal root ganglion (DRG) neurons. NO donors, sodium nitroprusside (SNP) and S-nitro-N-acetylpenicillamine (SNAP), decreased capsaicin-evoked currents (Icap). NO scavengers, hemoglobin and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (CPTIO), prevented the inhibitory effect of SNP on Icap. Membrane-permeable cGMP analogs, 8-bromoguanosine 3', 5'-cyclic monophosphate (8bromo-cGMP) and 8-(4chlorophenylthio)-guanosine 3',5'-cyclic monophosphate (8-pCPT-cGMP), and the guanylyl cyclase stimulator YC-1 mimicked the effect of SNP on Icap. The PKG inhibitor KT5823 prevented the inhibition of Icap by SNP. These results suggest that NO can downregulate the function of TRPV1 through activation of the cGMP/PKG pathway in peripheral sensory neurons.
Keyword
MeSH Terms
-
Animals
Benzoates
Carbazoles
Cyclic GMP-Dependent Protein Kinases
Ganglia, Spinal
Guanosine
Guanylate Cyclase
Hemoglobins
Humans
Imidazoles
Neurons
Nitric Oxide
Nitroprusside
Penicillamine
Phosphotransferases
Proteins
Rats
Receptors, Cytoplasmic and Nuclear
Sensory Receptor Cells
Spinal Nerve Roots
Tissue Donors
Benzoates
Carbazoles
Cyclic GMP-Dependent Protein Kinases
Guanosine
Guanylate Cyclase
Hemoglobins
Imidazoles
Nitric Oxide
Nitroprusside
Penicillamine
Phosphotransferases
Proteins
Receptors, Cytoplasmic and Nuclear
Figure
Cited by 2 articles
-
Regulation of Ca2+ Signaling in Pulmonary Hypertension
Amy L. Firth, Jun Yeon Won, Won Sun Park
Korean J Physiol Pharmacol. 2013;17(1):1-8. doi: 10.4196/kjpp.2013.17.1.1.TRPV1 in Salivary Gland Epithelial Cells Is Not Involved in Salivary Secretion via Transcellular Pathway
Seulki Choi, Yong-Hwan Shin, Eun Namkoong, Sung-Min Hwang, Xin Cong, Guangyan Yu, Kyungpyo Park
Korean J Physiol Pharmacol. 2014;18(6):525-530. doi: 10.4196/kjpp.2014.18.6.525.
Reference
-
1. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389:816–824. PMID: 9349813.
Article2. Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998; 21:531–543. PMID: 9768840.
Article3. Oh U, Hwang SW, Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J Neurosci. 1996; 16:1659–1667. PMID: 8774434.
Article4. Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000; 288:306–313. PMID: 10764638.
Article5. Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000; 405:183–187. PMID: 10821274.
Article6. Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA. 2002; 99:10150–10155. PMID: 12097645.
Article7. Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007; 27:2331–2337. PMID: 17329430.8. Lopshire JC, Nicol GD. Activation and recovery of the PGE2-mediated sensitization of the capsaicin response in rat sensory neurons. J Neurophysiol. 1997; 78:3154–3164. PMID: 9405535.9. Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci USA. 2005; 102:4536–4541. PMID: 15764707.
Article10. Levine JD, Alessandri-Haber N. TRP channels: targets for the relief of pain. Biochim Biophys Acta. 2007; 1772:989–1003. PMID: 17321113.
Article11. Vyklický L, Lyfenko A, Susánková K, Teisinger J, Vlachová V. Reducing agent dithiothreitol facilitates activity of the capsaicin receptor VR-1. Neuroscience. 2002; 111:435–441. PMID: 12031340.
Article12. Jin Y, Kim DK, Khil LY, Oh U, Kim J, Kwak J. Thimerosal decreases TRPV1 activity by oxidation of extracellular sulfhydryl residues. Neurosci Lett. 2004; 369:250–255. PMID: 15464274.
Article13. Tousova K, Susankova K, Teisinger J, Vyklicky L, Vlachova V. Oxidizing reagent copper-o-phenanthroline is an open channel blocker of the vanilloid receptor TRPV1. Neuropharmacology. 2004; 47:273–285. PMID: 15223306.
Article14. Blaise GA, Gauvin D, Gangal M, Authier S. Nitric oxide, cell signaling and cell death. Toxicology. 2005; 208:177–192. PMID: 15691583.
Article15. Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003; 54:469–487. PMID: 14726604.16. Lee JJ. Nitric oxide modulation of GABAergic synaptic transmission in mechanically isolated rat auditory cortical neurons. Korean J Physiol Pharmacol. 2009; 13:461–467. PMID: 20054493.
Article17. Martínez-Ruiz A, Cadenas S, Lamas S. Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic Biol Med. 2011; 51:17–29. PMID: 21549190.
Article18. Ahern GP, Hsu SF, Jackson MB. Direct actions of nitric oxide on rat neurohypophysial K+ channels. J Physiol. 1999; 520:165–176. PMID: 10517809.19. Castel H, Vaudry H. Nitric oxide directly activates GABA(A) receptor function through a cGMP/protein kinase-independent pathway in frog pituitary melanotrophs. J Neuroendocrinol. 2001; 13:695–705. PMID: 11489086.
Article20. Lang RJ, Harvey JR, McPhee GJ, Klemm MF. Nitric oxide and thiol reagent modulation of Ca2+-activated K+ (BKCa) channels in myocytes of the guinea-pig taenia caeci. J Physiol. 2000; 525:363–376. PMID: 10835040.21. Renganathan M, Cummins TR, Waxman SG. Nitric oxide blocks fast, slow, and persistent Na+ channels in C-type DRG neurons by S-nitrosylation. J Neurophysiol. 2002; 87:761–775. PMID: 11826045.22. Fukao M, Mason HS, Britton FC, Kenyon JL, Horowitz B, Keef KD. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J Biol Chem. 1999; 274:10927–10935. PMID: 10196172.23. Han J, Kim N, Kim E, Ho WK, Earm YE. Modulation of ATP-sensitive potassium channels by cGMP-dependent protein kinase in rabbit ventricular myocytes. J Biol Chem. 2001; 276:22140–22147. PMID: 11303020.
Article24. Herring N, Rigg L, Terrar DA, Paterson DJ. NO-cGMP pathway increases the hyperpolarisation-activated current, If, and heart rate during adrenergic stimulation. Cardiovasc Res. 2001; 52:446–453. PMID: 11738061.25. Yoshimura N, Seki S, de Groat WC. Nitric oxide modulates Ca2+ channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol. 2001; 86:304–311. PMID: 11431511.26. Zhou XB, Ruth P, Schlossmann J, Hofmann F, Korth M. Protein phosphatase 2A is essential for the activation of Ca2+-activated K+ currents by cGMP-dependent protein kinase in tracheal smooth muscle and Chinese hamster ovary cells. J Biol Chem. 1996; 271:19760–19767. PMID: 8702682.27. Zsombok A, Schrofner S, Hermann A, Kerschbaum HH. A cGMP-dependent cascade enhances an L-type-like Ca2+ current in identified snail neurons. Brain Res. 2005; 1032:70–76. PMID: 15680943.28. Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One. 2009; 4:e7596. PMID: 19893614.
Article29. Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006; 2:596–607. PMID: 16998480.30. Núñez L, Vaquero M, Gómez R, Caballero R, Mateos-Cáceres P, Macaya C, Iriepa I, Gálvez E, López-Farré A, Tamargo J, Delpón E. Nitric oxide blocks hKv1.5 channels by S-nitrosylation and by a cyclic GMP-dependent mechanism. Cardiovasc Res. 2006; 72:80–89. PMID: 16876149.31. Almanza A, Navarrete F, Vega R, Soto E. Modulation of voltage-gated Ca2+ current in vestibular hair cells by nitric oxide. J Neurophysiol. 2007; 97:1188–1195. PMID: 17182910.32. Irwin C, Roberts W, Naseem KM. Nitric oxide inhibits platelet adhesion to collagen through cGMP-dependent and independent mechanisms: the potential role for S-nitrosylation. Platelets. 2009; 20:478–486. PMID: 19852686.33. Jian K, Chen M, Cao X, Zhu XH, Fung ML, Gao TM. Nitric oxide modulation of voltage-gated calcium current by S-nitrosylation and cGMP pathway in cultured rat hippocampal neurons. Biochem Biophys Res Commun. 2007; 359:481–485. PMID: 17544367.34. Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997; 17:3525–3537. PMID: 9133377.
Article35. Thippeswamy T, McKay JS, Quinn JP, Morris R. Nitric oxide, a biological double-faced janus--is this good or bad? Histol Histopathol. 2006; 21:445–458. PMID: 16437390.36. Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol. 1996; 108:277–293. PMID: 8894977.37. Ellershaw DC, Greenwood IA, Large WA. Dual modulation of swelling-activated chloride current by NO and NO donors in rabbit portal vein myocytes. J Physiol. 2000; 528:15–24. PMID: 11018102.
Article38. White RE, Lee AB, Shcherbatko AD, Lincoln TM, Schonbrunn A, Armstrong DL. Potassium channel stimulation by natriuretic peptides through cGMP-dependent dephosphorylation. Nature. 1993; 361:263–266. PMID: 7678699.
Article39. Dun NJ, Dun SL, Forstermann U, Tseng LF. Nitric oxide synthase immunoreactivity in rat spinal cord. Neurosci Lett. 1992; 147:217–220. PMID: 1283459.
Article40. Zhang X, Verge V, Wiesenfeld-Hallin Z, Ju G, Bredt D, Synder SH, Hökfelt T. Nitric oxide synthase-like immunoreactivity in lumbar dorsal root ganglia and spinal cord of rat and monkey and effect of peripheral axotomy. J Comp Neurol. 1993; 335:563–575. PMID: 7693774.
Article41. Saito S, Kidd GJ, Trapp BD, Dawson TM, Bredt DS, Wilson DA, Traystman RJ, Snyder SH, Hanley DF. Rat spinal cord neurons contain nitric oxide synthase. Neuroscience. 1994; 59:447–456. PMID: 7516502.
Article42. Haley JE, Dickenson AH, Schachter M. Electrophysiological evidence for a role of nitric oxide in prolonged chemical nociception in the rat. Neuropharmacology. 1992; 31:251–258. PMID: 1630593.
Article43. Malmberg AB, Yaksh TL. Spinal nitric oxide synthesis inhibition blocks NMDA-induced thermal hyperalgesia and produces antinociception in the formalin test in rats. Pain. 1993; 54:291–300. PMID: 8233543.
Article44. Przewłocki R, Machelska H, Przewłocka B. Inhibition of nitric oxide synthase enhances morphine antinociception in the rat spinal cord. Life Sci. 1993; 53:PL1–PL5. PMID: 7685846.
Article45. Hao JX, Xu XJ. Treatment of a chronic allodynia-like response in spinally injured rats: effects of systemically administered nitric oxide synthase inhibitors. Pain. 1996; 66:313–319. PMID: 8880855.
Article46. Roche AK, Cook M, Wilcox GL, Kajander KC. A nitric oxide synthesis inhibitor (L-NAME) reduces licking behavior and Fos-labeling in the spinal cord of rats during formalin-induced inflammation. Pain. 1996; 66:331–341. PMID: 8880857.
Article47. Machelska H, Labuz D, Przewłocki R, Przewłocka B. Inhibition of nitric oxide synthase enhances antinociception mediated by mu, delta and kappa opioid receptors in acute and prolonged pain in the rat spinal cord. J Pharmacol Exp Ther. 1997; 282:977–984. PMID: 9262366.48. Aley KO, McCarter G, Levine JD. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J Neurosci. 1998; 18:7008–7014. PMID: 9712669.
Article49. Ferreira SH, Duarte ID, Lorenzetti BB. The molecular mechanism of action of peripheral morphine analgesia: stimulation of the cGMP system via nitric oxide release. Eur J Pharmacol. 1991; 201:121–122. PMID: 1665419.
Article50. Ferreira SH, Lorenzetti BB, Faccioli LH. Blockade of hyperalgesia and neurogenic oedema by topical application of nitroglycerin. Eur J Pharmacol. 1992; 217:207–209. PMID: 1425939.
Article51. Harima A, Shimizu H, Takagi H. Analgesic effect of L-arginine in patients with persistent pain. Eur Neuropsychopharmacol. 1991; 1:529–533. PMID: 1822318.
Article52. Moore PK, Oluyomi AO, Babbedge RC, Wallace P, Hart SL. L-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse. Br J Pharmacol. 1991; 102:198–202. PMID: 2043923.
Article53. Kawabata A, Fukuzumi Y, Fukushima Y, Takagi H. Antinociceptive effect of L-arginine on the carrageenin-induced hyperalgesia of the rat: possible involvement of central opioidergic systems. Eur J Pharmacol. 1992; 218:153–158. PMID: 1327824.
Article54. Lauretti GR, Lima IC, Reis MP, Prado WA, Pereira NL. Oral ketamine and transdermal nitroglycerin as analgesic adjuvants to oral morphine therapy for cancer pain management. Anesthesiology. 1999; 90:1528–1533. PMID: 10360847.
Article55. Durate ID, Lorenzetti BB, Ferreira SH. Peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Eur J Pharmacol. 1990; 186:289–293. PMID: 1981187.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study of the Pathway of Nitric Oxide Production by Nitroglycerin in Trabecular Meshwork Cells
- Expression of vesicular glutamate transporter in transient receptor potential vanilloid 1-positive neurons in the rat trigeminal ganglion
- Capsaicin Blocks the Hyperpolarization-Activated Inward Currents via TRPV1 in the Rat Dorsal Root Ganglion Neurons
- Pathogenesis of Cerebral Vasospasm
- The Study for Isoforms of Nitric Oxide Synthase in the Rat Penis and Major Pelvic Ganglion