Korean J Physiol Pharmacol.
2012 Jun;16(3):187-192. 10.4196/kjpp.2012.16.3.187.
Hop Extract Produces Antinociception by Acting on Opioid System in Mice
- Affiliations
-
- 1Department of Pharmacology, Institute of Natural Medicine, College of Medicine, Hallym University, Chuncheon 200-702, Korea. hwsuh@hallym.ac.kr
- KMID: 1768012
- DOI: http://doi.org/10.4196/kjpp.2012.16.3.187
Abstract
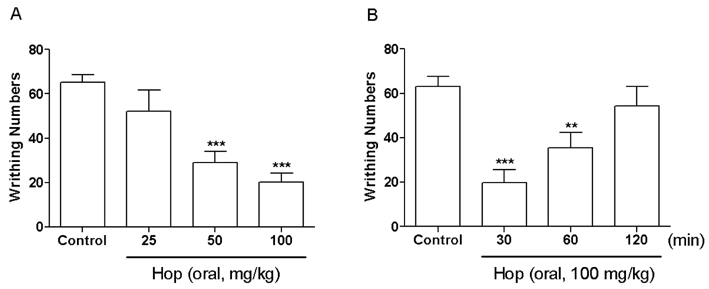
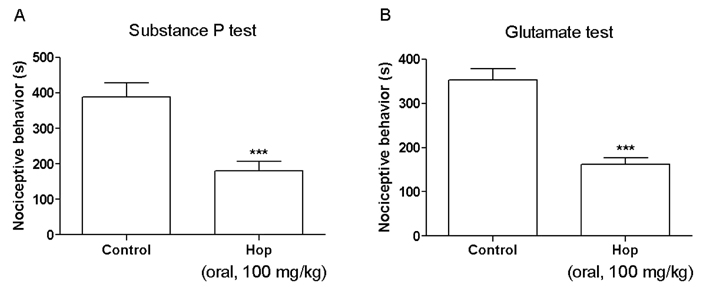
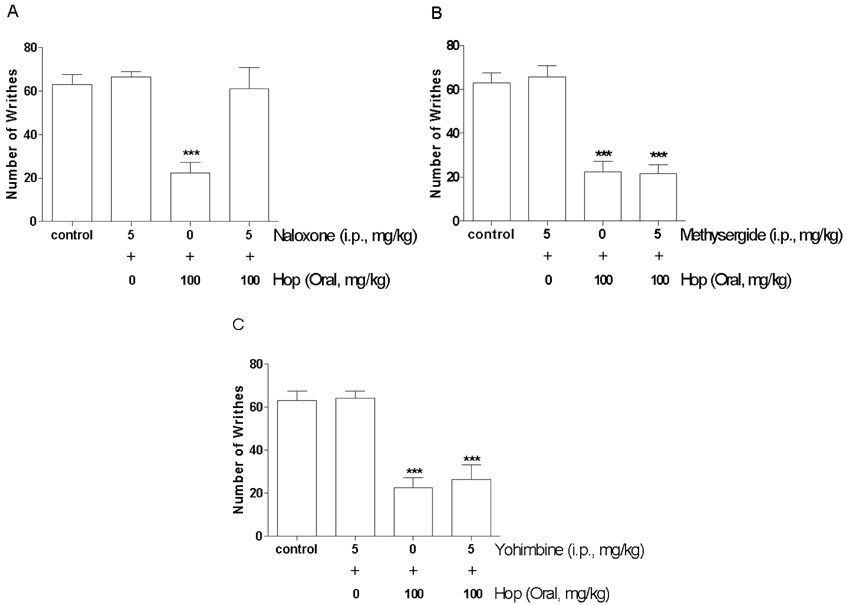
- In the present study, the antinociceptive profiles of hop extract were characterized in ICR mice. Hop extract administered orally (from 25 to 100 mg/kg) showed an antinociceptive effect in a dose-dependent manner as measured in the acetic acid-induced writhing test. Antinociceptive action of hop extract was maintained at least for 60 min. Moreover, cumulative response time of nociceptive behaviors induced with intraplantar formalin injection was reduced by hop extract treatment during the 2nd phases. Furthermore, the cumulative nociceptive response time for intrathecal injection of substance P (0.7 microg) or glutamate (20 microg) was diminished by hop extract. Intraperitoneal pretreatment with naloxone (an opioid receptor antagonist) attenuated antinociceptive effect induced by hop extract in the writhing test. However, methysergide (a 5-HT serotonergic receptor antagonist) or yohimbine (an alpha2-adrenergic receptor antagonist) did not affect antinociception induced by hop extract in the writhing test. Our results suggest that hop extract shows an antinociceptive property in various pain models. Furthermore, the antinociceptive effect of hop extract may be mediated by opioidergic receptors, but not serotonergic and alpha2-adrenergic receptors.
Keyword
MeSH Terms
Figure
Reference
-
1. Salvador RL. Hops. Can Pharm J. 1994. 127:203–204.2. Sakamoto K, Konings WN. Beer spoilage bacteria and hop resistance. Int J Food Microbiol. 2003. 89:105–124.3. Zuurbier KWM, Fung SY, Scheffer JJC, Verpoorte R. Formation of aromatic intermediates in the biosynthesis of bitter acids in Humulus Lupulus. II Phytochemistry. 1995. 38:77–82.4. Blumenthal M. . The Complete German Commission E Monograph: Therapeutic Guide to Herbal Medicines. 1998. Boston, Austin, U.S.A.: American Botanical Council, Integrative Medicine Communications;147.5. Grieve M. A Modern Herbal. 1971. New York, Raven, U.S.A.: Dover Publications Inc..6. Weiss RF. Herbal Medicine. 1988. Gothenburg, Sweden.: Ab Arcanum;285–286.7. Yamamoto K, Wang J, Yamamoto S, Tobe H. Suppression of cyclooxygenase-2 gene transcription by humulon of beer hop extract studied with reference to glucocorticoid. FEBS Lett. 2000. 465:103–106.8. Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980. 67:313–316.9. Hylden JL, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981. 217:212–215.10. Koster R, Anderson M, Beer EJ. Acetic acid for analgesic screening. Federal Proceeding. 1959. 18:412.11. Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 1985. 14:69–76.12. Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987. 30:103–114.13. Choi SS, Han KJ, Lee JK, Lee HK, Han EJ, Kim DH, Suh HW. Antinociceptive mechanisms of orally administered decursinol in the mouse. Life Sci. 2003. 73:471–485.14. Park SH, Sim YB, Choi SM, Seo YJ, Kwon MS, Lee JK, Suh HW. Antinociceptive profiles and mechanisms of orally administered vanillin in the mice. Arch Pharm Res. 2009. 32:1643–1649.15. Park SH, Sim YB, Han PL, Lee JK, Suh HW. Antidepressant-like effect of chlorogenic acid isolated from Artemisia capillaris Thunb. Animal Cells Syst. 2010. 14:253–259.16. Suh HW, Song DK, Son KH, Wie MB, Lee KH, Jung KY, Do JC, Kim YH. Antinociceptive mechanisms of dipsacus saponin C administered intracerebroventricularly in the mouse. Gen Pharmacol. 1996. 27:1167–1172.17. Suh HW, Song DK, Kim YH. Differential effects of adenosine receptor antagonists injected intrathecally on antinociception induced by morphine and beta-endorphin administered intracerebroventricularly in the mouse. Neuropeptides. 1997. 31:339–344.18. Suh HW, Chung KM, Kim YH, Huh SO, Song DK. Effects of histamine receptor antagonists injected intrathecally on antinociception induced by opioids administered intracerebroventricularly in the mouse. Neuropeptides. 1999. 33:121–129.19. Takashi M, Matsuyama M, Furuhashi K, Kodama Y, Shinzato M, Shamoto M, Nakashima N. Composite tumor of mucinous cystadenoma and somatostatinoma of the kidney. Int J Urol. 2003. 10:603–606.20. Vyklicky L. Bonica JJ, Liebeskind JC, Albe-Fessard DG, editors. The techniques for the study of pain in animals. Advances in pain research and theraphy. 1979. New York, U.S.A.: Raven;727–745.21. Satyanarayana PS, Jain NK, Singh A, Kulkarni SK. Isobolographic analysis of interaction between cyclooxygenase inhibitors and tramadol in acetic acid-induced writhing in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004. 28:641–649.22. Cervero F, Laird JM. Visceral pain. Lancet. 1999. 353:2145–2148.23. Ulugöl A, Ozyigit F, Yesilyurt O, Dogrul A. The additive antinociceptive interaction between WIN 55,212-2, a cannabinoid agonist, and ketorolac. Anesth Analg. 2006. 102:443–447.24. Choi SS, Lee JK, Suh HW. Antinociceptive profiles of aspirin and acetaminophen in formalin, substance P and glutamate pain models. Brain Res. 2001. 921:233–239.25. Chung KM, Lee KC, Choi SS, Suh HW. Differential roles of spinal cholera toxin- and pertussis toxin-sensitive G proteins in nociceptive responses caused by formalin, capsaicin, and substance P in mice. Brain Res Bull. 2001. 54:537–542.26. Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996. 64:345–355.27. Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test: characteristic biphasic pain response. Pain. 1989. 38:347–352.28. Cumberbatch MJ, Herrero JF, Headley PM. Exposure of rat spinal neurones to NMDA, AMPA and kainate produces only short-term enhancements of responses to noxious and nonnoxious stimuli. Neurosci Lett. 1994. 181:98–102.29. Schmauss C, Yaksh TL. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984. 228:1–12.30. Yaksh TL. Direct evidence that spinal serotonin and noradrenaline terminals mediate the spinal antinociceptive effects of morphine in the periaqueductal gray. Brain Res. 1979. 160:180–185.31. Yaksh TL. Multiple opioid receptor systems in brain and spinal cord: Part I. Eur J Anaesthesiol. 1984. 1:171–199.32. Jensen TS, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res. 1984. 321:287–297.33. Wigdor S, Wilcox GL. Central and systemic morphine-induced antinociception in mice: contribution of descending serotonergic and noradrenergic pathways. J Pharmacol Exp Ther. 1987. 242:90–95.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antinociception Effect and Mechanisms of Campanula Punctata Extract in the Mouse
- Effect of Agrimonia pilosa Ledeb Extract on the Antinociception and Mechanisms in Mouse
- Roles of Opioid Receptor Subtype in the Spinal Antinociception of Selective Cyclooxygenase 2 Inhibitor
- Capsaicin Reduces Ethanol Consumption in C57BL/6 but not DBA/2 Mice
- Effects of Whole Body Irradiation on Morphine, DAMGO, DPDPE, U50,488H and beta-endorphin-Induced Antinociception