Korean J Physiol Pharmacol.
2010 Oct;14(5):285-289. 10.4196/kjpp.2010.14.5.285.
Antinociception Effect and Mechanisms of Campanula Punctata Extract in the Mouse
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Hallym University, Chuncheon 200-702, Korea. hwsuh@hallym.ac.kr
- 2Institute of Natural Medicine, College of Medicine, Hallym University, Chuncheon 200-702, Korea.
- KMID: 2071695
- DOI: http://doi.org/10.4196/kjpp.2010.14.5.285
Abstract
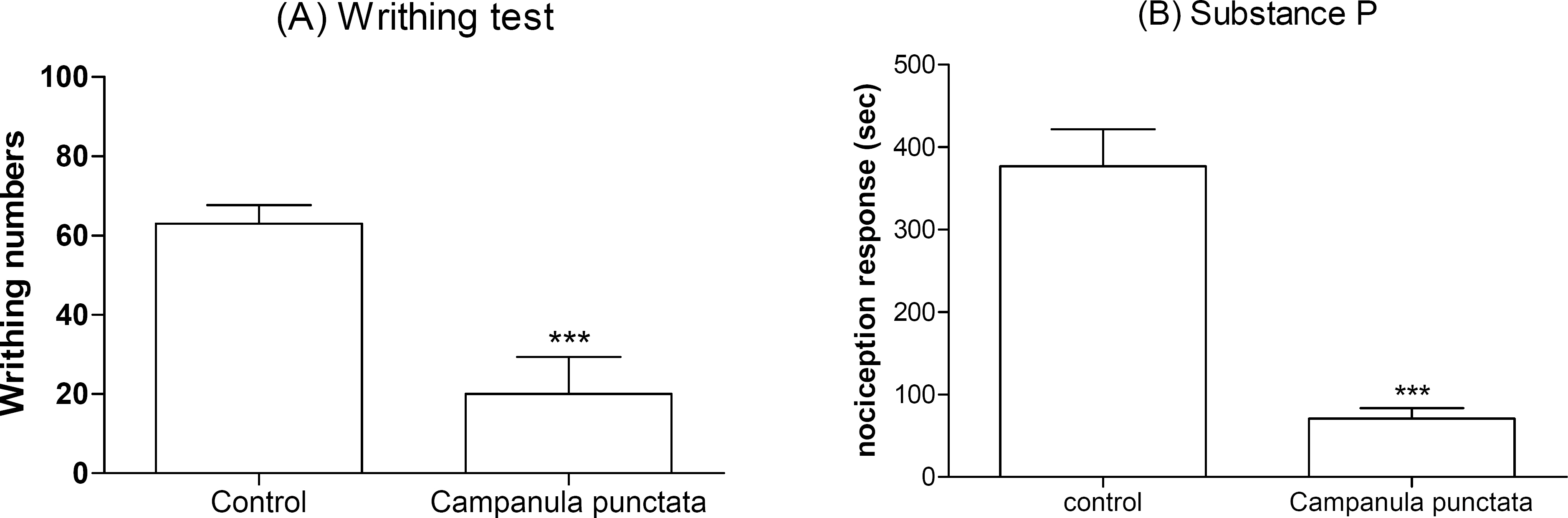
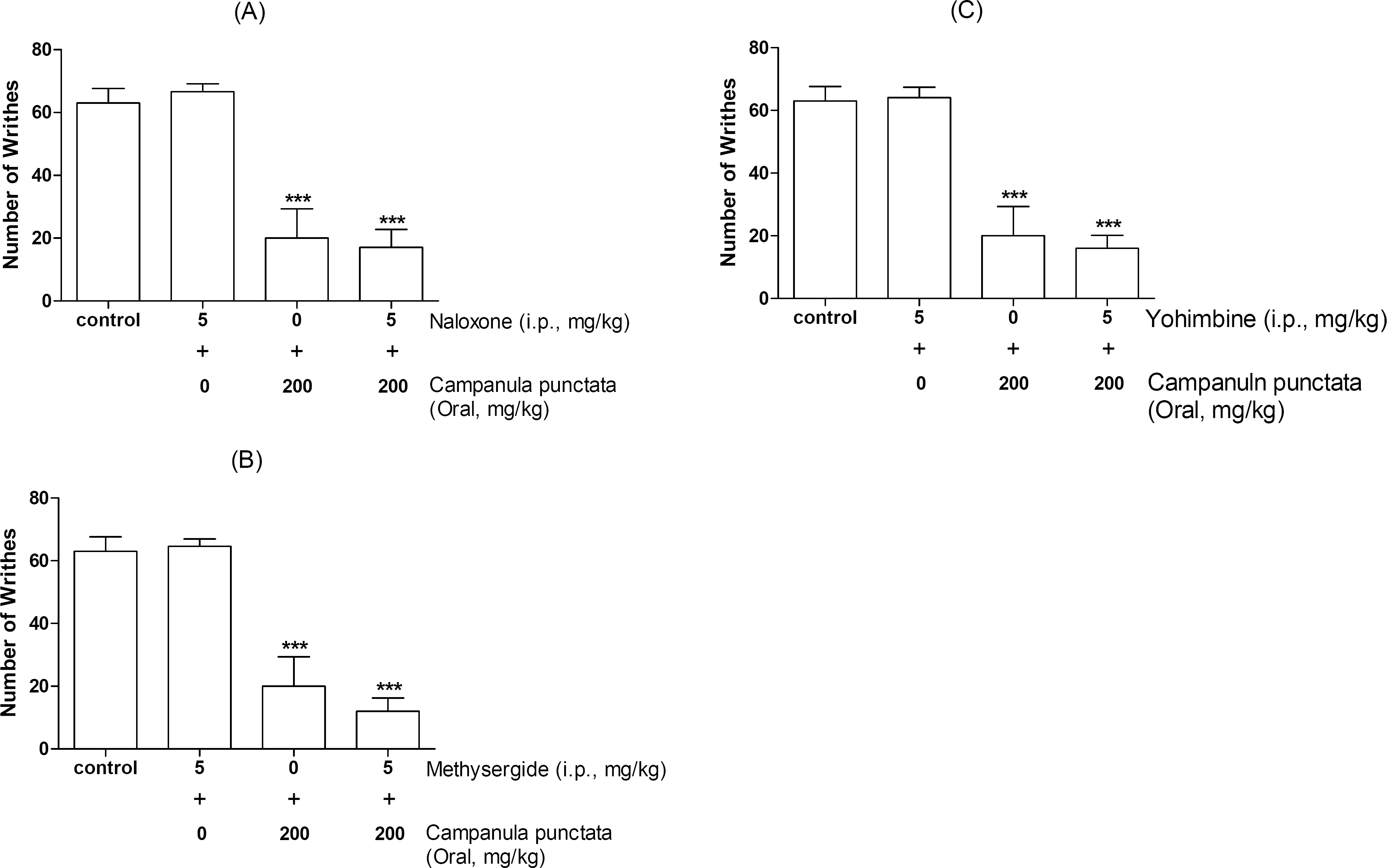
- In the present study, the antinociceptive profiles of Campanula punctata extract were examined in ICR mice. The Campanula punctata contain a large dose of saponin. Campanula punctata extract administered orally (200 mg/kg) showed an antinociceptive effect as measured by the tail-flick and hot-plate tests. In addition, Campanula punctata extract attenuated the writhing numbers in the acetic acid-induced writhing test. Furthermore, the cumulative nociceptive response time for intrathecal (i.t.) injection of substance P (0.7 microgram) was diminished by Campanula punctata extract. Intraperitoneal (i.p.) pretreatment with yohimbine (alpha2-adrenergic receptor antagonist) attenuated antinociceptive effect induced by Campanula punctata extract in the writhing test. However, naloxone (opioid receptor antagonist) or methysergide (5-HT serotonergic receptor antagonist) did not affect antinociception induced by Campanula punctata extract in the writhing test. Our results suggest that Campanula punctata extract shows an antinociceptive property in various pain models. Furthermore, this antinociceptive effect of Campanula punctata extract may be mediated by alpha2-adrenergic receptor, but not opioidergic and serotonergic receptors.
MeSH Terms
Figure
Reference
-
References
1. Kathleen N, Brenzel ED. Sunset Western Garden Book. 6th ed.Sunset Publishing Corporation;1995. p. 606–607.2. Shin CY, Lee WJ, Lee EB, Choi EY, Ko KH. Platycodin D and D3 increase airway mucin release in vivo and in vitro in rats and hamsters. Planta Med. 2002; 68:221–225.
Article3. Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980; 67:313–316.
Article4. Hylden JL, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981; 217:212–215.
Article5. D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941; 72:74–79.6. Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953; 107:385–393.7. Koster R, Anderson M, Beer EJ. Acetic acid for analgesic screening. Federal Proceeding. 1959; 18:412.8. Choi SS, Han KJ, Lee JK, Lee HK, Han EJ, Kim DH, Suh HW. Antinociceptive mechanisms of orally administered decursinol in the mouse. Life Sci. 2003; 73:471–485.
Article9. Park SH, Sim YB, Choi SM, Seo YJ, Kwon MS, Lee JK, Suh HW. Antinociceptive Profiles and Mechanisms of Orally Administered Vanillin in the Mice. Arch Pharm Res. 2009; 32:1643–1649.
Article10. Suh HW, Song DK, Son KH, Wie MB, Lee KH, Jung KY, Do JC, Kim YH. Antinociceptive mechanisms of dipsacus saponin C administered intracerebroventricularly in the mouse. General Pharmacology. 1996; 27:1167–1172.
Article11. Suh HW, Song DK, Kim YH. Differntial effects of adenosine receptor antagonist injected intrathecally on antinociception induced by morphine and beta-endorphin administered intra-cerebroventricularly in the mouse. Neuropeptides. 1997; 31:339–344.12. Suh HW, Chung KM, Kim YH, Huh SO, Song DK. Effect of histamine receptor antagonists injected intrathecally on antinociception induced by opioids administered intracerebroventricularly in the mouse. Neuropeptides. 1999; 33:121–129.13. Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985; 22:1–31.
Article14. Grumbach L. The prediction of analgesic activity in man by animal testing. Knighton RS, Dumke PR, editors. Pain. Boston: Little Brown and Co;1966. p. 163–182.15. Vyklicky L. The techniques for the study of pain in animals. Bonica JJ, Liebeskind JC, Albe-Fessard DG, editors. Adv Pain Res Ther. Vol. 3. New York: Raven.;1979. p. 727–745.16. Cumberbatch MJ, Herrero JH, Headley PM. Exposure of rat spinal neurons to NMDA, AMPA and kainate produces only short-term enhancements of responses to noxious and non-noxious stimuli. Neuroscience Letters. 1994; 181:98–102.17. Schmauss C, Yaksh TL. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral chemical and cutaneous thermal stimuli in the rat. J Pharmacol Exp Ther. 1984; 228:1–12.18. Yaksh TL. Direct evidence that spinal serotonin and noradrenaline terminals mediate the spinal antinpciceptive effects of morphine in the periaqueductal gray. Brain Res. 1979; 160:180–185.19. Yaksh TL. Multiple opioid receptor systems in brain and spinal cord: Part I. Eur J Anaesthesiol. 1984; 1:171–199.20. Jensen TS, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Research. 1984; 321:287–297.
Article21. Wigdor S, Wilcox GL. Central and systemic morphine-induced antinociception in mice: contribution of descending serotonergic and noradrenergic pathways. Journal of pharmacology and Experimental Therapeutics. 1987; 242:90–95.